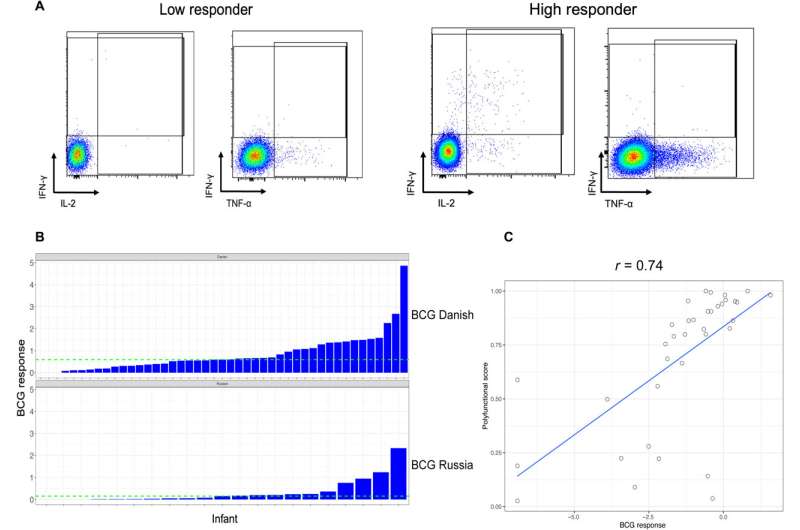
The Bacillus Calmette-Guerin vaccine against tuberculosis can elicit a good response in neonates. Infants who are exposed to HIV but are uninfected display an altered immunity to vaccination.
In a new study published in Science Advances, Donald D. Nyangahu and a research team in molecular medicine and human genetics at the University of Washington Seattle, U.S., and the University of Cape Town, decoração festa infantil urso marrom e azul South Africa, hypothesized that the infant pioneer gut microbiota affects vaccine T cell responses. The team analyzed BCG-immune responses at 7 weeks of age and categorized the responses as high or low. The study outcomes highlighted a causal role of B. infantis in the early life of antigen-specific immunity.
The gut microbiota
The gut microbiota is a crucial determinant of metabolism and immune development, where the infant pioneer microbiota is largely determined by the mother, affecting the welfare of the offspring. The Bacille-Calmette Guerin vaccine is administered at birth to millions of infants as a prototypical neonatal T cell vaccine to improve immunity.
Interestingly, studies have shown how infants who are exposed to HIV but are uninfected have an altered gut microbiome compared to infants who are HIV unexposed. To examine the effect of BCG responses in the uninfected yet exposed to HIV infants, the team studied the key microbiota of high versus low BCG responders early in life among the study cohorts. The researchers next studied germ-free and pathogen-free neonatal mouse models to understand causative relationships between the gut microbiota and the BCG vaccine to influence immunogenicity.
The T cell response to BCG
The team measured the BCG response to T cells after neonatal vaccinations in infants exposed yet uninfected by HIV. The results were highly variable, the researchers measured them as the frequency of CD4+ cytokine production after stimulation. They classified the infants with BCG responses above the median as high responders and the remainder as low responders. They did not observe differences in the median gestational age, maternal age or birth weight, although they noted a significant response between infant sex and the vaccine response.
Nyangahu and colleagues classified the high versus low BCG responses with different gut microbiota compositions and noted the diverse response on immune cell populations in infant mice thereafter. At first, they studied the variations in the BCG response across all infants relative to their diverse gut microbiota and profiled them from infants with HIV exposure without infection up to the first week of life using deep sequencing.
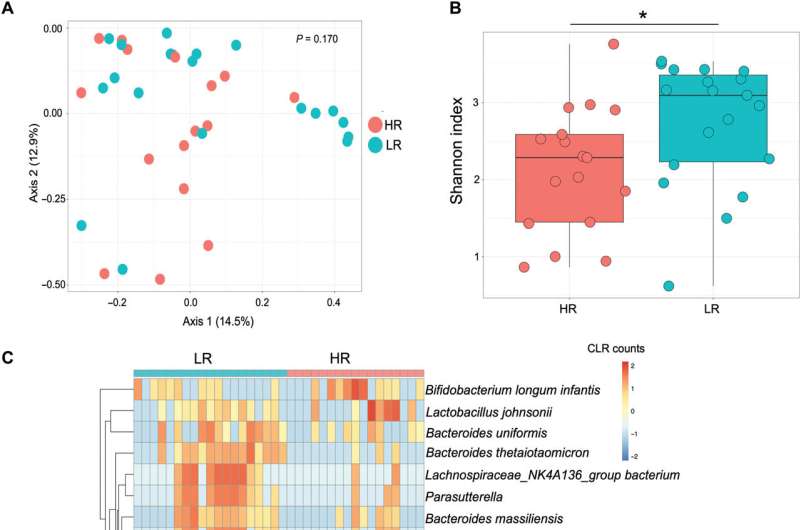
The scientists used metagenome sequencing to identify the diverse and abundant taxa between high responders and low responders to BCG. The outcomes showed high levels of Bifidobacterium longum subspecies in the high responders compared to the low-responders. The low responders had significantly higher levels of Bacteroides thetaiotaomicron. When the scientists conducted quantitative polymerase chain reaction on DNA isolated from stools of infants, they noted B. infantis to be more abundant in the high responders. The scientists studied the influence of infant gut microbiota on immune development using a germ-free mouse model. They noted the expression of total memory CD4 T cells to be significantly higher in the high-responder and low-responder samples, with potential associations with BCG T cell responses.
The early immunity response in specific-pathogen free and germ-free mouse models
The team tested the differential bacteria identified in human infants and their impacts on immune development early in life in neonatal germ-free or specific-pathogen free murine models. The bacterial strains of B. infantis and B. thetaiotaomicron have been previously associated with immunoregulation and inflammation in murine studies. However, the effect of these bacteria on the development of immunity during infancy are unknown.
Nyangahu and team investigated this by mono-colonizing germ-free mice with B. infantis, and B. thetaiotaomicron. The inflammatory and noninflammatory monocytes were both significantly reduced in the B. infantis and B. thetaiotaomicron mono-colonized mice. The scientists repeated the experiments in specific-pathogen free mice to observe significantly reduced body weight in those administered with B. infantis compared to B. thetaiotaomicron. Much like the germ-free variant, specific pathogen free mice had significantly higher frequencies of central memory CD4 T cells. The combined outcomes showed how B. infantis and B. thetaiotaomicron differentially affected the developing immune system in neonatal mice without causing inflammation.
Additional experiments
The research team additionally observed how B. infantis enhanced antigen-specific T cells after BCG vaccination in the germ-free and specific-pathogen free mice. Further experiments revealed the influence of B. infantis and B. thetaiotaomicron on the internal metabolome, and its impact on the colonic transcriptome in mice.
Inspired by these outcomes observed in mice, Nyangahu and colleagues studied how the impact of B. infantis and B. thetaiotaomicron affected the transcriptome in human colonic cells. While B. infantis lead to the upregulation of genes involved in antigen processing and presentation to foster immune tolerance in colonic epithelial cells cultured in the lab. B. infantis secreted proteins and not metabolites to induce a molecular signature similar to live bacteria.
Outlook
In this way, Donald D. Nyangahu and colleagues studied the susceptibility of neonates to infection with a poor response to most vaccines. The findings showed how the gut microbiota of early life influenced immune development, including antigen-specific T-cell responses to neonatal vaccination. While infants who responded well to neonatal vaccination had higher abundance of B. infantis, alongside relatively deficient B. thetaiotaomicron in the low responders. The colonization of B. infantis included a distinct transcriptome and metabolome signature early in life, which extended to human colonic epithelial cells.
The scientists identified B. infantis as a potential therapeutic intervention for improved T cell immunity and vaccine responsiveness in neonates. Since the bacterial strain is already available as a live biotherapeutic, future studies can examine its influence on vaccine responses in human infants.
More information:
Donald D. Nyangahu et al, Bifidobacterium infantis associates with T cell immunity in human infants and is sufficient to enhance antigen-specific T cells in mice, Science Advances (2023). DOI: 10.1126/sciadv.ade1370
Journal information:
Science Advances
Source: Read Full Article