Dr. Akhilesh K. Gaharwar, associate professor, has developed a highly printable bioink as a platform to generate anatomical-scale functional tissues. This study was recently published in the American Chemical Society’s Applied Materials and Interfaces.
Bioprinting is an emerging additive manufacturing approach that takes biomaterials such as hydrogels and combines them with cells and growth factors, which are then printed to create tissue-like structures that imitate natural tissues.
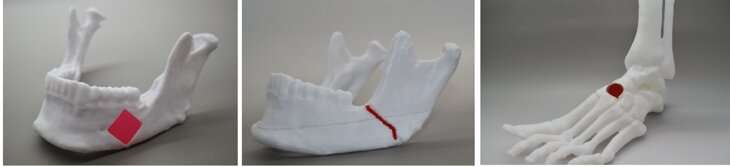
One application of this technology could be designing patient-specific bone grafts, an area that is gaining interest from researchers and clinicians. Managing bone defects and injuries through traditional treatments tends to be slow and expensive. Gaharwar said that developing replacement bone tissues could create exciting new treatments for patients suffering from arthritis, bone fractures, dental infections and craniofacial defects.
Bioprinting requires cell-laden biomaterials that can flow through a nozzle like a liquid, but solidify almost as soon as they’re deposited. These bioinks need to act as both cell carriers and structural components, requiring them to be highly printable while providing a robust and cell-friendly microenvironment. However, current bioinks lack sufficient biocompatibility, printability, structural stability and tissue-specific functions needed to translate this technology to preclinical and clinal applications.
To address this issue, Gaharwar’s research group is leading efforts in developing advanced bioinks known as Nanoengineered Ionic-Covalent Entanglement (NICE) bioinks. NICE bioinks are a combination of two reinforcement techniques (nonreinforcement and ionic-covalent network), which together provide more effective reinforcement that results in much stronger structures.

Once bioprinting is complete, the cell-laden NICE networks are crosslinked to form stronger scaffolds. This technique has allowed the lab to produce full-scale, cell-friendly reconstructions of human body parts, including ears, blood vessels, cartilage and even bone segments.
Soon after the bioprinting, the enclosed cells start depositing new proteins rich in a cartilage-like extracellular matrix that subsequently calcifies to form a mineralized bone over a three-month period. Almost 5 percent of these printed scaffolds consisted of calcium, which is similar to cancellous bone, the network of spongy tissue typically found in vertebral bones.
To understand how these bioprinted structures induce stem cell differentiation, a next-generation genomics technique called whole transcriptome sequencing (RNA-seq) was utilized. RNA-seq takes a snapshot of all genetic communication inside the cell at given moment. The team worked with Dr. Irtisha Singh from Texas A&M Health Science Center, who served as a co-investigator.
“The next milestone in 3-D bioprinting is the maturation of bioprinted constructs toward the generation of functional tissues,” Gaharwar said. “Our study demonstrates that NICE bioink developed in our lab can be used to engineer 3-D-functional bone tissues.”
Source: Read Full Article
