The unprecedented development of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines over the past 12 months appears to be mitigating the COVID-19 epidemic in geographies where implementation has been successful. The rollout of over a dozen vaccines, with many more in the pipeline, has brought hope that the pandemic can be brought to an end and life can soon return to normal.
Reduction in influenza temporary
Non-pharmaceutical interventions (NPIs), such as lockdowns, mask-wearing, acyclovir cream 5 cold sores social distancing, and closure of non-essential institutions, have led to a temporary reduction in cases and deaths in most regions where they were applied. Along with the steep fall in COVID-19 cases, seasonal influenza has also shown a drastic decline during the pandemic.
However, as vaccine uptake becomes more robust globally, social and business interactions are bound to increase, and the flu will come back, say researchers. As antigenic drift occurs, new strains will emerge, including potentially novel strains. This could, perhaps, lead to a new pandemic of influenza.
Seasonal flu claims around 400,000 lives every year, on average, but four significant influenza pandemics have already occurred over the last century. The 1918-1919 H1N1 Spanish flu with 50 million deaths, 1957-1959 H2N2 Asian flu with 1.5 million deaths, 1968-1969 H3N2 Hong Kong flu with 1 million deaths, and 2009-2010 H1N1pdm09 swine flu with somewhere 150,000 to 575,000 deaths worldwide.
Combination vaccine components
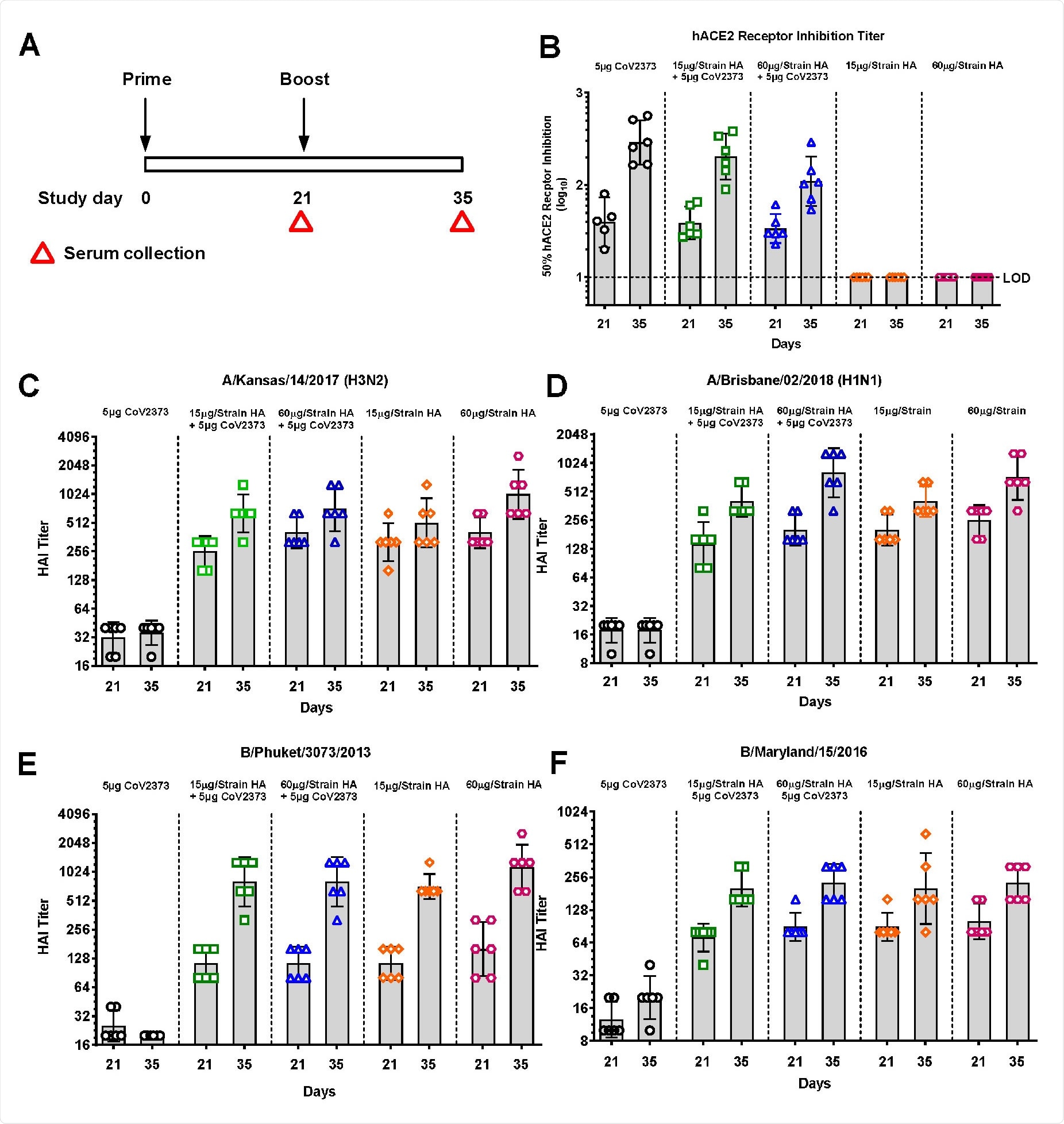
Now, in new research posted to the bioRxiv* preprint server, scientists at Novavax, Inc. present a vaccine including recombinant influenza hemagglutinin (HA) antigen along with recombinant SARS-CoV-2 spike protein, with saponin Matrix-M adjuvant. Both have passed independent safety tests in phase 1 trials.
Quadrivalent nanoparticle influenza vaccine (qNIV) has repeatedly been shown to elicit broadly protective immunity against older and newly emerging strains, probably due to highly conserved neutralizing epitopes in the HA stem domain and rudimentary esterase domain. Both hemagglutination inhibition (HAI) and microneutralizing (MN) antibodies have been elicited against both older and novel A and B strains of H3N2 and H1N1 viruses.
Earlier researchers employed a recombinant insect cell production platform for the generation of nanoparticles bearing the full-length SARS-CoV-2 spike stabilized in the prefusion conformation. These proteins are in the form of trimers that form rosettes by associating with polysorbate 80 (PS 80) micelles.
The antibodies elicited by this vaccine were protective against the Wuhan-Hu-1-like virus USA-WA1, as well as the UK (B.1.1.7) and South African (B.1.351) variants of SARS-CoV-2. When tested in mice and two monkey models, the vaccine prevented both upper and lower respiratory tract infections after exposure to this virus.
The vaccines’ safety, tolerability, and immunogenicity have been established in many trials, showing it to induce a Th1-biased CD4+ T cell response.
Thus, both components of this vaccine have been individually tested for safety and efficacy.
Appeal of combination vaccine
In the current study, the qNIV and spike vaccine combination was shown to retain its protective capabilities against influenza A and B, via HAI and neutralizing antibodies, while also protecting against both upper and lower respiratory infection with SARS-CoV-2. The researchers took advantage of this unique pandemic situation coupled with the data from the qNIV vaccine trial to develop this novel combination.
While earlier vaccines were almost always intended for use in children, the current vaccine offers a low-risk pathway for approval since the influenza HA component has been shown to elicit protective levels of immunity, as well as the recombinant spike protein component.
Protection against emerging variants
In addition, the combination vaccine offers protection against emerging SARS-CoV-2 variants, which is an essential requirement in the current situation. Viruses like this one, with a ribonucleic acid (RNA) genome, are known to mutate rapidly and often, especially when faced with immune responses from host cells and organisms, and in the case of zoonoses like SARS-CoV-2, due to antigenic shift as well.
The South African variant of SARS-CoV-2 has shown adaptation to immune challenges by escape mutations which enabled recurrent outbreaks among a population group supposed to have achieved population immunity. The use of nanoparticle-based spike protein allows for cryptic epitopes to be exposed, taking advantage of epitopes conserved between this variant and the prototype strain.
This could include eliciting antibodies that are not obtained by natural infection.
The influenza vaccine also appeared to show poor efficacy on the field, as shown by the occurrence of several seasonal outbreaks of severe influenza mainly due to A(H3N2) strains. This is apparently due to differences in the antigens when generated in the egg-based vaccines, but also due to antigenic changes in the viruses.
However, the Matrix-M adjuvanted recombinant hemagglutinin (HA) trivalent nanoparticle influenza vaccine (tNIV) was recently shown to induce immunity to highly conserved epitopes with broad neutralizing capability.
The use of the full-length surface HA glycoprotein allows the wild-type circulating viral sequence to be retained while simultaneously allowing cryptic epitopes to be exposed to the immune system and thus elicit polyclonal broadly neutralizing antibodies.
Earlier studies showed that this formulation did improve the immune HAI response against A/H3N2 variants resulting from antigenic drift over several years, compared to the high-dose inactivated trivalent influenza vaccine (IIV3-HD).
What are the implications?
Not only is the suppression of influenza likely to be transient, but co-infections of both influenza and SARS-CoV-2 will become more common. Influenza will often be overlooked due to the very similar symptoms of COVID-19.
Admitting that the outcome of such co-infections is unknown, it is urgent to prepare for this eventuality by a combination vaccine such as this. Not only does it obviate the need for separate vaccine logistics, but it protects against current and emerging strains of both viruses.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Source
- Massare, M. J. et al. (2021). Combination Respiratory Vaccine Containing Recombinant SARS-CoV-2 Spike and Quadrivalent Seasonal Influenza Hemagglutinin Nanoparticles with Matrix-M Adjuvant. bioRxiv preprint. doi: https://doi.org/10.1101/2021.05.05.442782; https://www.biorxiv.org/content/10.1101/2021.05.05.442782v1
Posted in: Drug Trial News | Medical Research News | Disease/Infection News
Tags: Animal Model, Antibodies, Antigen, Asian Flu, CD4, Cell, Cell Production, Children, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Flu, Genome, Glycoprotein, H1N1, H2N2, H3N2, Immune System, Immunization, Influenza, Nanoparticle, Nanoparticles, Pandemic, Protein, Research, Respiratory, Respiratory Tract Infections, Ribonucleic Acid, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Swine Flu, Syndrome, Vaccine, Virus

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article
