In a new study published in Cell Stem Cell, a team led by USC Stem Cell scientist Michael Bonaguidi, Ph.D., demonstrates that neural stem cells—the stem cells of the nervous system—age rapidly.
“There is chronological aging, and there is biological aging, and they are not the same thing, lithium carbonate side affects ” said Bonaguidi, an Assistant Professor of Stem Cell Biology and Regenerative Medicine, Gerontology and Biomedical Engineering at the Keck School of Medicine of USC. “We’re interested in the biological aging of neural stem cells, which are particularly vulnerable to the ravages of time. This has implications for the normal cognitive decline that most of us experience as we grow older, as well as for dementia, Alzheimer’s disease, epilepsy and brain injury.”
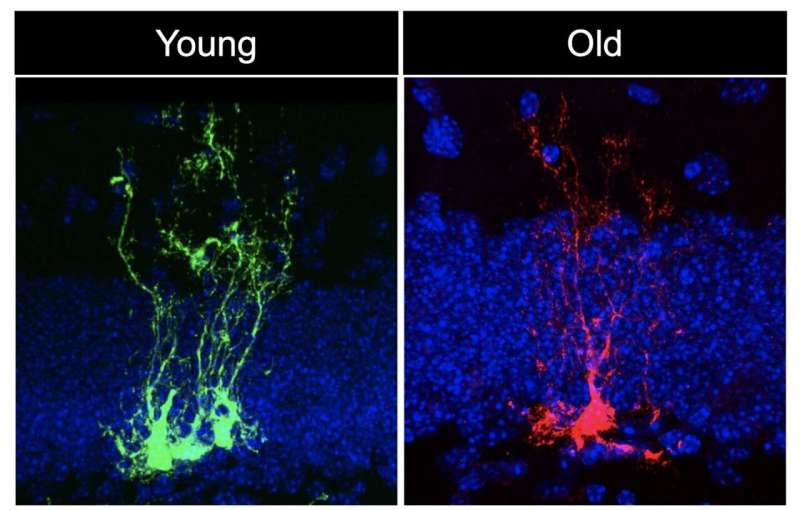
In the study, first author Albina Ibrayeva, a Ph.D. candidate in the Bonaguidi Lab in the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC, joined her colleagues in looking at the brains of young, middle-aged and old mice.
By tracing individual neural stem cells, or NSCs, over the course of several months, they identified “short-term NSCs” that quickly differentiate into more specialized neurons, and “long-term NSCs” that continually divide and replicate themselves to maintain an ongoing reserve of stem cells with the ability to generate many different cell types in the brain. This key population of long-term NSCs divided less often and failed to maintain their numbers as the mice aged.
The scientists next examined thousands of genes in the long-term NSCs, which were dividing less often and had slipped into an inactive state known as quiescence. The gene activity of the quiescent NSCs varied greatly in young versus middle-aged animals. As expected, there were changes in genes that control how long-term NSCs divide, as well as generate new neurons and other brain cells. Remarkably, there were many important changes in gene activity related to biological aging at younger ages than anticipated. These pro-aging genes make it more difficult for cells to repair damage to their DNA, regulate their genetic activity, control inflammation and handle other stresses.
Among the pro-aging genes, the scientists were most intrigued by Abl1, which formed the hub of a network of interrelated genes.
“We were interested in the gene Abl1, because no one has ever studied its role in neural stem cell biology—whether in development or in aging,” said Ibrayeva.
Using an existing, FDA-approved chemotherapy drug called Imatinib, scientists could easily inhibit the activity of the gene Abl1. The scientists gave older mice doses of Imatinib for six days. After the drug blocked the activity of the gene Abl1, the NSCs began to divide more and proliferate in the hippocampus, the part of the brain responsible for learning and memory.
Source: Read Full Article
