Researchers in the United States and Canada have identified DNA on/off “switches” within the angiotensin-converting enzyme 2 (ACE2) gene that may control the expression of this host cell receptor before, during and after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The SARS-CoV-2 virus is the agent responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic that has caused 174 million infections and more than 3.76 million deaths globally since the outbreak began in late December 2019.
The human ACE2 receptor is the main host cell structure that the SARS-CoV-2 surface spike protein binds to as the initial stage of the infection process.
The team – from Harvard University in Cambridge, Massachusetts, the University of Waterloo in Oregon, 2009 pharmacy tech day and McMaster University in Hamilton, Oregon – say that the DNA or intronic switches they identified appear to be responsive to immunologic and oxidative stress.
“Our work supports the further pursuit of these putative mechanisms in our understanding, prevention, and treatment of infection and disease caused by ACE2-utilizing viruses such as SARS-CoV, SARS-CoV-2, and future emerging SARS-related viruses,” writes Terence Capellini.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
More about the ACE2 receptor and SARS-CoV-2 infection
The ACE2 protein is a key catalytic regulator of the renin-angiotensin system (RAS) that controls blood pressure and maintains fluid homeostasis. The regulation of RAS by ACE2 inhibits the damaging effects of RAS overactivation such as vasoconstriction, fibrosis, and excessive inflammation.
The RAS system functions across multiple organs and ACE2 is expressed throughout the body, where it exerts protective effects on tissue function.
Changes in ACE2 expression following viral infection can lead to an imbalance in RAS signaling and contribute to the development of clinical symptoms such as excessive inflammation, myocardial injury, and lung injury.
Little is known about how ACE2 is turned on or off at the DNA level
Although great research efforts have been made to understand how SARS-CoV-2 interacts with ACE2, little is known about how ACE2 is turned on or off in human cells at the DNA level.
The team says that understanding the mechanisms that regulate ACE2 expression during viral infection is important from a pathology point of view, given that this may inhibit the protective effects of ACE2 activity.
“In addition, understanding the regulatory mechanisms controlling ACE2 expression prior to viral exposure may be of equal importance from a disease-prevention point of view, given that baseline levels of ACE2 in high-exposure tissues such as the lung may modify viral susceptibility,” writes Capellini and colleagues.
What did the study involve?
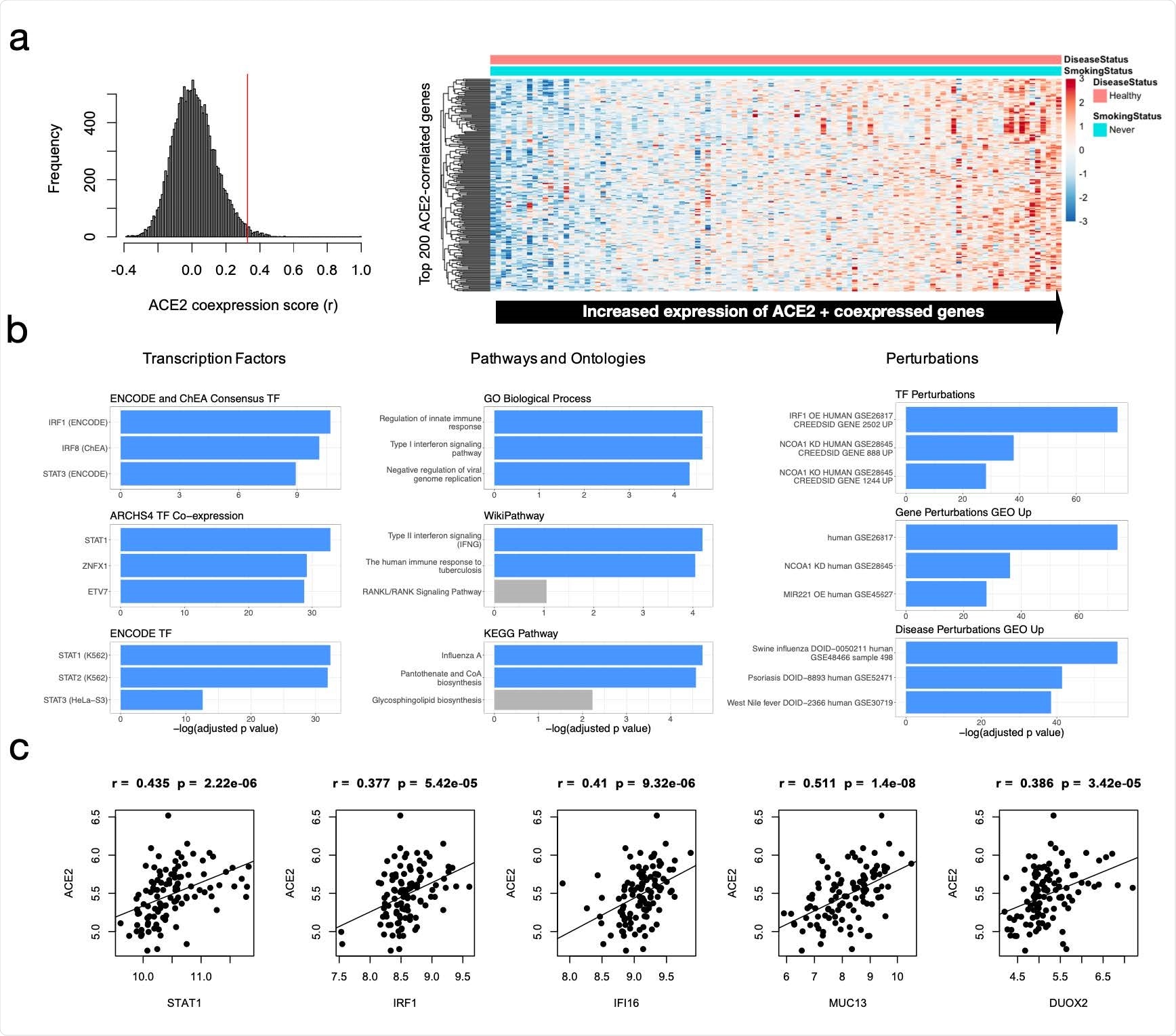
The team performed bioinformatic analyses of microarray expression datasets from healthy non-smokers and identified genes with expression patterns that significantly correlated with those of ACE2.
This enabled the researchers to generate hypotheses about ACE2 gene-regulatory mechanisms in the context of immune signaling and chronic oxidative stress.
Next, the team identifies putative non-coding regulatory elements within intronic regions of the ACE2 gene as potential determinants of ACE2 expression activity.
The researchers then considered cis-regulatory elements within the ACE2 locus, which may be mediators of this immune-response regulatory mechanism. After identifying six such regions, the team prioritized and experimentally deleted three putative intronic enhancers, which led to consistent down-regulation of ACE2 transcripts.
What are the implications of the study?
“We have indeed identified a number of DNA on/off switches for ACE2 that appear responsive to immunological and oxidative stress,” says Capellini and colleagues. “These switches may fine-tune how ACE2 is turned on or off before, during, and/or after infection by SARS-CoV-2 or other related coronaviruses.”
The researchers say the findings will help pave the way for additional functional studies of these switches and their potential therapeutic targeting in the future.
“We suggest that further experimental testing is warranted to confirm these predicted mechanisms, and furthermore, to develop potential strategies taking advantage of this knowledge to modify susceptibility and disease severity of coronavirus infections, particularly SARS-CoV-2,” concludes the team.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Capellini T, et al. Intronic regulation of SARS-CoV-2 receptor (ACE2) expression mediated by immune signaling and oxidative stress pathways. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.06.07.447351, https://www.biorxiv.org/content/10.1101/2021.06.07.447351v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Blood, Blood Pressure, Cell, Chronic, Coronavirus, Coronavirus Disease COVID-19, DNA, Enzyme, Fibrosis, Gene, Genes, Inflammation, Locus, Microarray, Oxidative Stress, Pandemic, Pathology, Protein, Receptor, Renin, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Stress, Syndrome, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article
