Contrary to the widely shared popular belief, the primary purpose of current vaccines against COVID-19 is solely to protect against serious forms of the disease in order to prevent the risk of hospitalizations and death… and not to block transmission between individuals. To do this, it would be necessary to aim for a so-called sterilizing vaccination: only then could the circle of contamination be interrupted, allowing to stop the pandemic.
How to reach this level of efficacy? In general, vaccination allows the induction of an immune response based on two types of cells: T lymphocytes, capable of destroying infected cells, acomplia half life and B lymphocytes which produce antibodies capable of neutralizing the virus (SARS-CoV-2 in the case of COVID) to prevent it from multiplying and infecting new healthy cells.
Current vaccines (including mRNA) are administered intramuscularly and are so called “systemic”: they allow the activation at the level of the whole body of a pool of immune cells, circulating by the blood, which can further reach the infected organs.
Effective as it is, this systemic immunity does not allow the mobilization of a high level of B and T lymphocytes in the nasal cavity and the lungs—to promote a rapid and effective protection against the virus—blocking it as soon as it arrives.
Conversely, an intranasal vaccination induces not only a systemic but also a local immune response—directly, therefore, at the gate of the entry of SARS-CoV-2. The local activation of the immune cells of the nasal mucosa would thus make it possible to overcome the race against time between the virus (which multiplies in our respiratory system) and our systemic immune system (which need to be mobilized to the infected site). Concretely this mucosal vaccination would quickly stop the virus and block its possibilities of dissemination and replication in our organism and thus avoid transmission and contamination.
The specifics of nasal vaccination
First observation, as we said, only the nasal vaccination can act directly at the level of entry of the virus. But we should also mention that the immune cells it activates there (resident T and B lymphocytes in the nose, mouth and upper respiratory tract) differ from those activated by the conventional (i.e., systemic) intramuscular vaccination. Moreover: nasal vaccination induces B lymphocytes that produce particular antibodies, IgA (Type A immunoglobulins), which are only very weakly induced by the intramuscular route—which mainly induces B cells producing IgG (Type G immunoglobulins).
Worth noting: IgAs have a greater capacity than IgGs to “capture” viruses in order to neutralize them. Another advantage of IgAs: they are more “versatile” than IgGs and thus can retain a significant level of effectiveness despite possible variations in the virus. For all these reasons, “mucosal” vaccination would prevent even moderate forms of the disease and block inter-individual transmission, to achieve sterilizing immunity.
The FluMist vaccine is the only example of intranasal vaccine available in human health that has received marketing authorization (AMM). This flu vaccine is based on an attenuated form of the causative virus (influenza), approved in the United States and Europe, and has an efficacy that surpasses that of the intramuscular vaccine in young children. However, its effectiveness is lower in adults owing to their already acquired mucosal immunity, due to previously contracted infections. Indeed, consisting of an attenuated version of the virus, the vaccine is quickly blocked by the local immunity in place reacting against the original virus: which leaves it less likely to act efficiently.
New nasal vaccine approach
Our research team (BioMAP laboratory, Joint University-INRAE ISP 1282 research unit), led by Professor Isabelle Dimier-Poisson, has recognized experience in immunology and mucosal vaccination. Based on our expertise, we have worked on an innovative anti-COVID mucosal vaccine strategy in order to deal with its multiple specificities. Our vaccine candidate is based on three innovations:
- The antigen: target of the virus, it represents the heart of the vaccine. It is an original fusion protein, designed in our laboratory, composed of the now-famous Spike (S) protein associated with another protein of the virus, the nucleoprotein (N). This fusion strategy allows our vaccine to maintain its efficacy against different variants since it targets some conserved parts of the virus, whatever the variations of the S protein.
- To optimize the activation of the mucosal immune response, our antigen is wrapped in “nano-carriers”. These nano-carriers are solely composed of sugar polymeric molecules, able to confer original properties of adhesion to the mucous membrane allowing an optimal administration of our protein. In this manner there is no need for an adjuvant (likely to create inflammation), thus reducing the risk of side effects.
- Finally, the last key element, a dedicated delivery system, or spray, capable of effectively depositing our vaccine in the nasal cavity, precisely at the level of the areas containing the mucosal immune cells.
Nasal anti-COVID vaccination: where are we?
Other teams are following this type of approach to develop anti-COVID vaccine delivered by the mucosal route. However, there are still very few vaccine candidates available in humans. We can mention two recent examples (September 2022), in China and India—bearing in mind that they do not use the intranasal route in the strict sense. A first vaccine, currently being tested in China, is administered by inhalation. CanSino Biologics’ vaccine has been approved by Chinese health authorities as a booster dose to protect against symptoms of COVID-19. It is based, like its intradermal counterpart, on a recombinant adenovirus expressing the S protein of SARS-CoV-2 and is delivered using a nebuliser through the mouth. It therefore requires a specific medical device and, due to its viral nature, even attenuated, presents a risk of side effects such as pulmonary inflammation. The second has been approved by the Indian health authorities: iNCOVACC, developed by Bharat Biotech, for primary vaccination in two doses administered through the nose.
This nasal vaccine similarly uses a modified and attenuated adenovirus to deliver the Spike protein of SARS-CoV-2. This vaccine, created by Michael S. Diamond, David T. Curiel (University of Washington), was the subject of a recent publication presenting preclinical trials in chimpanzees, and showing promising results. However, this result is not representative of a “true” nasal vaccination since it uses a dual route of vaccination: intranasal and intrabronchial. In this manner the vaccine is administered to the lungs, with a potential risk of inflammation. This preclinical result should therefore be considered with caution. It’s also worth noting that neither China nor India has yet released the results of human clinical studies supporting their decision to approve these vaccines. Regardless of the considered candidate, the ability to administer the vaccine formulation effectively via the nasal route is a challenge.
AstraZeneca has just announced in October the disappointing results of its first clinical trials of a nasal vaccine. This nasally administered version of its injectable vaccine (developed with researchers at the University of Oxford) showed only weak antibody responses in the nasal mucosa. The explanation would be that a large part of the vaccine, which uses a deactivated virus, would not have reached its target and would have ended up largely in the digestive tract before it could activate the immune system of the mucous membranes. A key point, which is underlined by the team of this latest vaccine candidate, is the importance of the spray system. Vaccination by nasal administration clearly requires consideration to optimize the in situ product delivery.
Final steps for our vaccine candidate
Our vaccine candidate has given excellent results against multiple variants of SARS-CoV-2, with protection against the disease and significant limitation of its transmission, in gold standard preclinical models (mice and hamsters). Our objective is now to validate its efficacy during clinical trials in humans, scheduled for 2023. The challenge, to go from preclinical to human, is to obtain an effective immune response despite a different delivery of the vaccine: indeed, in the mouse and hamster models, the volume of vaccine used and its delivery using micropipette deposition in the nose induce immunization not restricted to the nasal cavity but also reaching the upper part of the lungs.
However, in humans, we want to stay at the level of the nasal cavity in order to limit the risks of an uncontrolled immune reaction that can lead to an excessively strong inflammatory reaction in the lung (“cytokine storms”): the objective is to optimize the stimulation of the nasal mucosal system only. To do this, we want our vaccine to be completely deposited in the critical areas of the nose: where the virus nests to infect and multiply, and where the immune cells that must respond to the vaccine are located (at the level of the NALT, or Nasal Associated Lymphoid Tissues, where IgA-producing B lymphocytes and T lymphocytes are concentrated).
Developing a spray system suitable for nasal vaccination
Small nuance: a vaccine spray is different from a classical therapeutic spray used for multiple and repeated use. It must deliver a single, very precise dose targeting the mucosal immune system. From the start of our project, we began to work on those absolute prerequisite and set up research and development collaborations with two companies specialized in intranasal delivery systems: Aptar pharma and Medspray. The potential effectiveness of the spray systems is assessed by two methods:
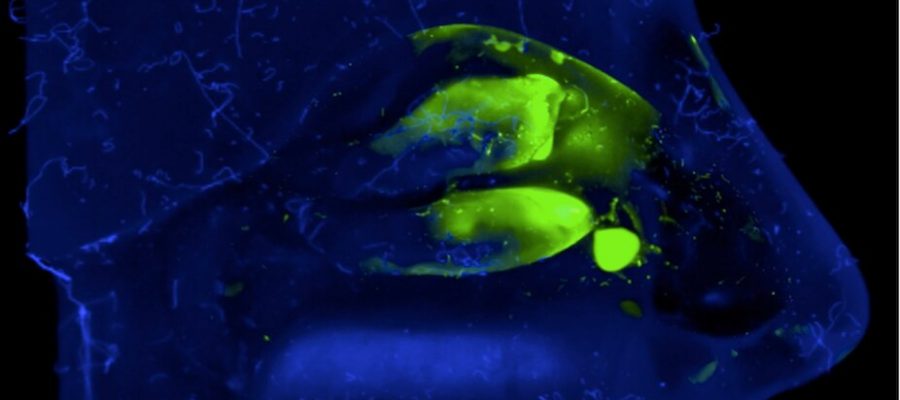
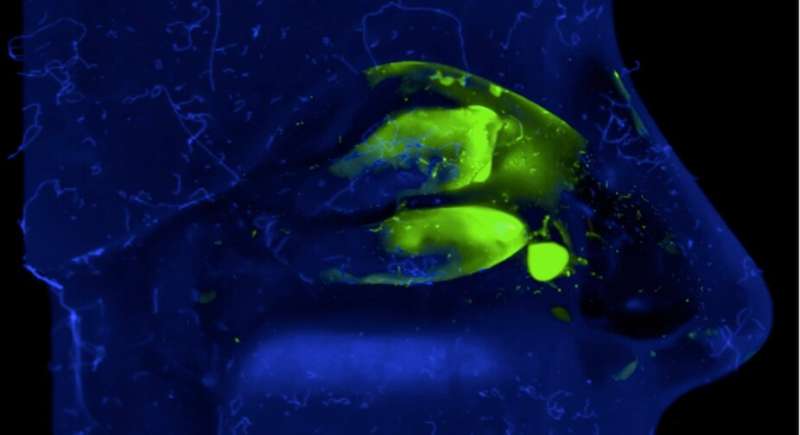
- In vitro, using an artificial model (nasal cast) which reproduces the human nasal cavity and makes it possible to assess, after adding a fluorescent dye, how our vaccine formulation is deposited in the different compartments. This model allows us to correct the spray to optimally target key immune system areas of the nasal mucosa.
- In vivo, we carry out comparative efficacy tests of the various spray systems in rabbits (among the gold standard animal model) to assess their ability to induce an optimal vaccine response in the nasal mucosa (in the context of regulatory toxicology tests).
Our goal is to select the optimal intranasal delivery system for future clinical trials, in terms of biological efficacy (maximal vaccination efficacy despite a delivery restricted to the nasal cavity). In addition, with the idea of designing a spray accessible to low-income countries, we are considering economic constraints to ensure that it can be mass-produced at reduced cost.
Provided by
The Conversation
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Source: Read Full Article