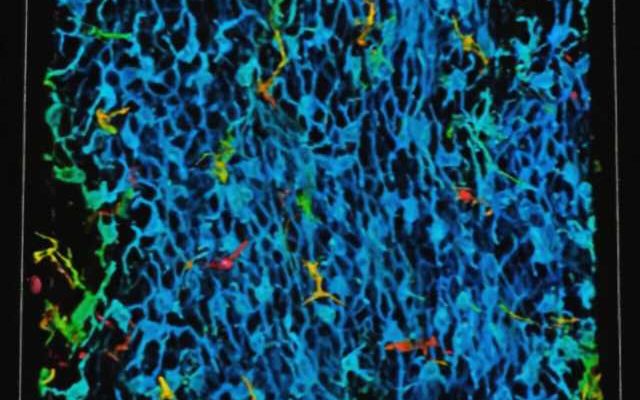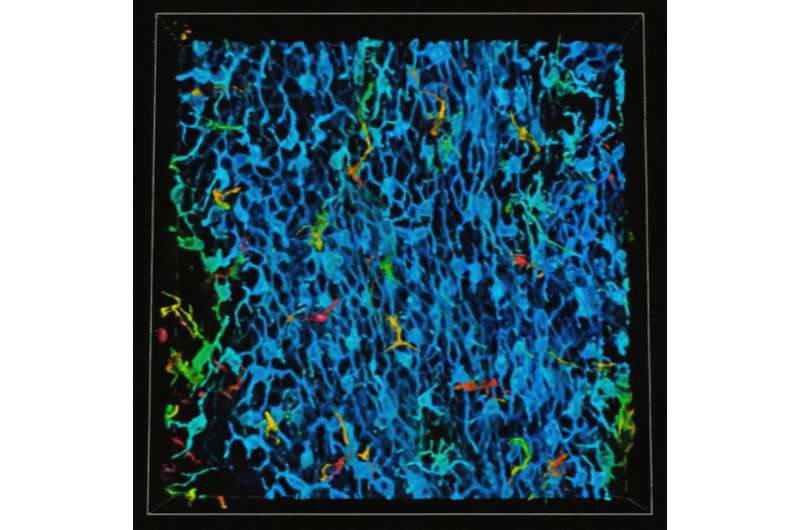
Investigators led by Kathleen Green, Ph.D., the Joseph L. Mayberry, Sr., Professor of Pathology and Toxicology, have discovered novel intercellular “crosstalk” between epidermal keratinocytes and melanoma cells that promote cancer growth and metastasis.
These signaling mechanisms could also serve as biomarkers for early cancer detection, according to the Northwestern Medicine study published in the Journal of Cell Biology.
More than 90,000 cases of melanoma, the most aggressive type of skin cancer, luvox and resperidal will be diagnosed this year alone, according to annual estimates from the American Cancer Society. While melanoma is less commonly diagnosed than other types of skin cancer, it is more likely to grow and metastasize if not detected early.
Melanoma cancer cells arise from melanocytes, pigmented cells located in the basal layer of the epidermis, the outermost layer of the skin. When exposed to ultraviolet (UV) radiation such as sunlight, melanocytes are triggered to produce pigment (commonly observed as tanning) through the activation of a signaling pathway involving melanocytes and keratinocytes—cells that surround melanocytes and act as a protective barrier to UV exposure.
Excessive UV exposure without proper protection can promote mutations in melanocytes, causing them to transform into cancerous melanoma cells. The mechanisms that keratinocytes and melanocytes utilize to interact with each other and promote the growth of melanoma tumor cells, however, have remained unclear.
A previous study from Green’s laboratory had initially found that the temporary loss of Desmoglein-1 (Dsg1)—a protein highly expressed in the upper layers of the epidermis that is temporarily lost in response to UV—controls signaling between melanocytes and keratinocytes to stimulate protective pigmentation. However, chronic loss of Dsg1 resulted in melanocytes rapidly spreading, a behavior similar to what is exhibited in early stages of melanoma development.
This discovery prompted further investigation for Green’s team to study and identify the signaling mechanisms, or “crosstalk,” between melanocytes and keratinocytes in melanoma cells.
“We were very interested in the crosstalk or the communication between these two cell types—highly abundant keratinocytes that surround each of these melanocytes, and the melanocytes which as they’re transitioning into melanoma cells, communicate back to the keratinocytes,” said Green, who is also a professor of Dermatology and a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Using RNA sequencing and immunofluorescence microscopy to study patient samples of melanoma cells, the investigators identified a melanocyte-keratinocyte communication loop between the two cell types which help the melanocytes changing into melanoma cells become more aggressive.
Essentially, the melanoma cells hijack a signaling pathway that normally turns off Dsg1 in response to UV by secreting specific factors that reduce the expression of Dsg1 in the surrounding keratinocytes, according to the authors. In response, this decrease of Dsg1 enhances the migratory behavior of melanoma cells and increases tumor progression.
“If the melanoma cells can keep Dsg-1 down, keep it at bay, they can move around more and then ultimately spread out of the epidermis to other places,” Green said.
Many individuals are predisposed to having many dysplastic nevi, or abnormal-looking moles that are not yet cancerous but have the potential to be. According to Green, identifying lower levels of Dsg1 could be a useful biomarker for determining whether these nevi could become cancerous in the future.
“To be able to detect whether or not these people also have a decrease [of Dsg1]in dysplastic nevi might help to predict if those dysplastic nevi are going to progress and could help physicians identify patients that are candidates for intervention therapies,” Green said.
Green added that her team is currently studying Dsg1 knockout in animal models of melanoma to determine how reduced levels of Dsg1 promotes melanoma initiation and progression. These studies will also help determine whether Dsg1 loss could be an effective biomarker for early detection and preventing tumor progression, according to Green.
“We firmly believe that understanding the pathways that are driving the initiation and the progression of early melanoma are important to understand,” Green said.
More information:
Hope E. Burks et al, Melanoma cells repress Desmoglein 1 in keratinocytes to promote tumor cell migration, Journal of Cell Biology (2023). DOI: 10.1083/jcb.202212031
Journal information:
Journal of Cell Biology
Source: Read Full Article