<img class="aligncenter" src="https://scx1.b-cdn.net/csz/news/800a/2023/lung-cancer-screening-1.jpg"
alt="Lung cancer screening guidelines perpetuate racial disparities, study finds"
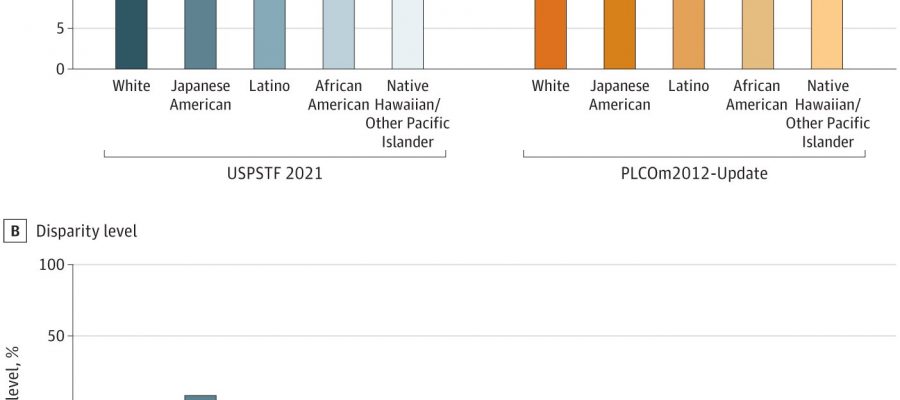
title="Racial Disparities in the Eligibility-Incidence (E-I) Ratio Through the US Preventive Services Task Force (USPSTF) 2021 and Risk-Based Screening (Prostate, Lung, Colorectal, can flagyl be used to treat a tooth infection and Ovarian Cancer Screening Trial 2012 [PLCOm2012]-Update Model 6-Year Risk ≥1.3%) Criteria. Credit: JAMA Oncology (2023). DOI: 10.1001/jamaoncol.2023.4447″ width=”800″ height=”530″>
Current national guidelines that rely on age and smoking exposure to recommend people for lung cancer screening are disproportionally failing minority populations including African Americans, according to a new study led by researchers at Stanford Medicine.
An alternative risk-based method that incorporates additional information including family history and other health problems such as previous cancer diagnoses does a better job of eliminating disparities among races, the study found.
The disparities persist despite a revision to the guidelines that was implemented in 2021 to address race-based disparities in screening eligibility.
“Our study shows that these changes to the guidelines are not sufficient to address race-based differences in lung cancer incidence and age at diagnosis,” said associate professor of neurosurgery and of biomedical informatics Summer Han, Ph.D. “This is a lost opportunity to detect lung cancers early when cancers are still treatable. Early detection saves lives.”
Han is the senior author of the study, which was published Oct. 26 in JAMA Oncology. Neurosurgery instructor Eunji Choi, Ph.D., is the lead author of the research.
Lung cancer is the leading cause of cancer death in the United States, killing about 127,000 people annually, but it can be treatable if detected early.
Low-dose computed tomography, or CT scan, has been shown to significantly reduce the number of lung cancer deaths. But because the radiation delivered by the scans can be harmful (they use on average about 10 times the radiation of standard X-rays), only those people at relatively high risk for lung cancer should be screened. The two biggest risk factors for lung cancer are exposure to tobacco smoke and age.
Screening guidelines
In 2013, the United States Preventive Services Task Force issued guidelines recommending annual low-dose CT scans for people aged 55 to 80 who had a minimum cumulative smoking exposure of 30 pack years (a pack year is the number of packs of 20 cigarettes smoked each day multiplied by the number of years the person has smoked) and who were still smoking or who had stopped smoking within the previous 15 years. Someone who smoked three packs per day for 10 years would have an exposure of 30 pack years, for example.
The task force is an independent panel of national experts in disease prevention and evidence-based medicine. Although doctors can make their own decisions about who should and should not be screened, they generally follow the task force’s guidelines.
In 2021, the task force revised these guidelines—lowering the starting age for screening to 50 years and the exposure levels to 20 pack years—to address the fact that African Americans tend to develop lung cancers at a younger age and after less smoking exposure than other racial groups.
“Many studies have found that only about 32% of African Americans with lung cancer, versus 56% of white people, were eligible for screening under the 2013 guidelines,” Han said.
Choi, Han and their colleagues analyzed a multi-ethnic group of 105,261 people 45 to 75 years old with a history of smoking who enrolled in a research study between 1993 and 1996. Participants were African American, Japanese American, Latino, Native Hawaiian and other Pacific Islander, or white.
When they enrolled, the participants filled out a questionnaire about their smoking history, sociodemographic factors such as education level and body mass index, and their medical background, including a personal history of cancer or a family history of lung cancer.
The researchers used a national cancer registry to identify which participants were diagnosed with lung cancer within six years of their enrollment in the study.
Using the answers to the questionnaire, the researchers assessed which of the participants would have been eligible for lung cancer screening under either the updated Preventive Services Task Force guidelines, which use only age and smoking history, or a risk-based assessment method that also uses information about each person’s family history, health background and any previous cancer diagnoses.
They found that overall, 24% of people in the study would have been eligible for screening based on the task force’s updated guidelines. But there were differences among the racial and ethnic groups: 30% of white people would have qualified for screening compared with 25.5% of Japanese Americans, 25.1% of Native Hawaiians and other Pacific Islanders, 21.4% of African Americans, and 15.7% of Latinos.
A better measure of disparities
Reduced eligibility doesn’t indicate a health inequity on its own. It’s possible that one group may have comparatively lower smoke exposure than another, or may be at lower or higher risk biologically, for example.
A more telling measure is a ratio researchers call eligibility-to-incidence rates—or the number of people in a group eligible for screening compared with the number of lung cancer cases found in that group over a certain time. Higher ratios imply adequate screening; lower ratios imply that some lung cancer cases are occurring in people who were not deemed eligible for screening.
When the researchers calculated this ratio using the 2021 task force guidelines, they found that white people in the study had an eligibility-to-incidence ratio of 20.3, while African Americans had a ratio of 9.5. This difference was driven by the fact that fewer African Americans were eligible for screening (21.4% versus 30.2%) and that African Americans had a higher incidence of lung cancer over the subsequent six years (2.2% versus 1.5% of white participants). Native Hawaiians and other Pacific Islanders had an eligibility-to-incidence ratio of 16.8 versus the 20.3 of white participants.
In contrast, the risk-based analysis did a better, but not perfect, job at eliminating disparities. Under this analysis, African Americans had an eligibility-to-incidence ratio of 15.9 versus 18.4 for white participants. The improvement was primarily due to an increase in the number of African Americans who would have been eligible for screening—35.7% versus the 21.4% eligible under the task force guidelines.
The difference in eligibility-to-incidence ratios between Native Hawaiians and other Pacific Islanders and white people also improved to 16.6 versus 18.4. Minimal differences were observed between white people and the other racial groups.
Further analysis showed that selecting people to screen based on the risk-based analysis was more likely than the task force guidelines to accurately identify people with lung cancer—a measure known as sensitivity—and fewer screens were needed to detect one case of lung cancer (26 versus 30).
The researchers hope that their findings will spur a national dialogue about race-based disparities in lung cancer screening recommendations and how to devise more equitable and effective guidelines.
“It’s critically important to identify high-risk people across racial and ethnic groups,” Han said.
“Our study shows that risk-based screening reduces racial disparities and improves screening efficiency. Disparities evident in the U.S. Preventive Services Task Force’s lung cancer screening guidelines may have a significant impact on lung cancer mortality in the United States. We clearly show that a method that incorporates additional information in screening assessments is better at identifying true cases of lung cancer. It also reduces the number of false positives.”
More information:
Eunji Choi et al, Risk Model–Based Lung Cancer Screening and Racial and Ethnic Disparities in the US, JAMA Oncology (2023). DOI: 10.1001/jamaoncol.2023.4447
Journal information:
JAMA Oncology
Source: Read Full Article