<img class="aligncenter" src="https://scx1.b-cdn.net/csz/news/800a/2023/researchers-find-a-cau.jpg"
alt="Researchers find a cause of Parkinson's disease"
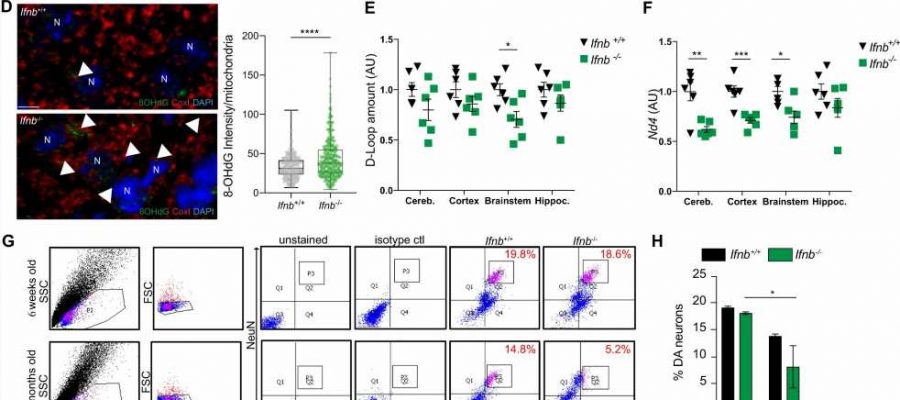
title="Complex I respiratory chain subunits are altered in PD patients and PD models (Ifnb-/-/Ifnar1-/-) due to mtDNA damages. A Enrichment plot of KEGG-OXPHOS from microarray data comparing sporadic Parkinson’s disease with dementia (sPDD/N = 13) and healthy controls (HC/N = 14). B Immunoblot showing loss of ND4 and ND5 proteins in the prefrontal cortex of healthy controls (HC) or Parkinson Disease patients (PD), with quantification. Pink indicates females and blue males. C Immunoblot showing loss of ND4 and ND5 proteins in the brainstems of 12-month-old mice, with quantifications. D Immunolabelling for 8-OHdG (pseudocoloured green), CoxI (mitochondria, depo testosterone hgh pseudocoloured red) in mice midbrains. DAPI stains nuclei. Scale bars equal 5 microns. Quantification of 8-OHdG as integrated density of 8-OHdG in mitochondria of 3-month-old mice. Each dot represents a mitochondrion assessed (N = 415) from 3 biological replicates. E qPCR for D-loop in different brain regions in 6-week-old mice. F qPCR for major arc based on Nd4 amplification in different brain regions in 6-week-old mice. For E and F each dot represents one individual animal, N = 6. G FACS cell sorting to isolate single dopaminergic (DN) neurons from midbrain of 6-week and 6-month-old mice. NeuN+ indicate neurons and NeuN+TH+, dopaminergic neurons. H Quantification of (G) in 6-week-old or 6-month-old mice. N = 3. I qPCR for D-loop in single isolated DN. Data are mean ± SEM, N = 3 mice/group. J Position of SVs and SNVs detected with MitoSV-seq in 6-week-old Ifnb−/− mice. Circos plots of mouse mtDNA genome displaying Heavy and Light Origin of replication (OH and OL, respectively), major arc and D-loop (outer blue circle). mtDNA genes are displayed in the middle circle (gray lines). SVs as arches with deletions (blue), tandem duplications, inversions and insertions (orange). Thickness of the arches correspond to SVs heteroplasmy. SNVs are marked with short red lines (¬_) according to their positions in mouse mtDNA. Intensity of red lines correspond to SNVs’ heteroplasmy. K Pie chart showing the distribution of variation types detected in Ifnb−/− mice; SNP: Single Nucleotide Polymorphism. For all graphs, * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 by unpaired t-test. Credit: Molecular Psychiatry (2023). DOI: 10.1038/s41380-023-02251-4″ width=”800″ height=”530″>
Until recently, our understanding of Parkinson’s disease has been quite limited, which has been apparent in the limited treatment options for management of this debilitating condition.
Our recent understanding has primarily revolved around the genetic factors responsible for familial cases, while the causative factors in the vast majority of patients remained unknown.
However, in a new study, researchers from the University of Copenhagen have unveiled new insights into the workings of the brain in Parkinson’s patients. Leading the discovery is Professor Shohreh Issazadeh-Navikas.
“For the first time, we can show that mitochondria, the vital energy producers within brain cells, particularly neurons, undergo damage, leading to disruptions in mitochondrial DNA. This initiates and spreads the disease like a wildfire through the brain,” says Issazadeh-Navikas and adds, “Our findings establish that the spread of the damaged genetic material, the mitochondrial DNA, causes the symptoms reminiscent of Parkinson’s disease and its progression to dementia.”
Parkinson’s disease is a chronic condition that affects the central nervous system, leading to symptoms such as difficulty walking, tremors, cognitive challenges, and, eventually, dementia.
The disease afflicts over 10 million people worldwide. While there is currently no cure, certain medical treatments can offer relief from its symptoms.
Small fragments of mitochondrial DNA spread the disease
By examining both human and mouse brains, researchers discovered that the damage to mitochondria in brain cells occurs and spreads when these cells have defects in anti-viral response genes. They sought to understand why this damage occurred and how it contributed to the disease.
Their search led to a remarkable revelation.
“Small fragments of—actually DNA—from the mitochondria are released into the cell. When these fragments of damaged DNA are misplaced, they become toxic to the cell, prompting nerve cells to expel this toxic mitochondrial DNA,” Issazadeh-Navikas explains.
“Given the interconnected nature of brain cells, these toxic DNA fragments spread to neighboring and distant cells, similar to an uncontrolled forest fire sparked by a casual bonfire,” she adds.
The dream is a blood sample
Issazadeh-Navikas envisions that this study marks the initial stride towards a better understanding of the disease, and the development of future treatments, diagnostics, and measurement of treatment efficacy for Parkinson’s disease.
She also expressed hope that “detecting the damaged mitochondrial DNA could serve as an early biomarker for disease development”.
Biomarkers are objective indicators of specific medical conditions observed in patients. While some biomarkers are common, such as blood pressure, body temperature and body mass index, others provide insights into particular diseases, like gene mutations in cancer or level of blood sugar for diabetes. Identifying a biomarker for Parkinson’s disease holds significant promise for enhancing future treatments.
“It could be possible that the damage of the mitochondrial DNA in the brain cells leaks from the brain into the blood. That would make it possible to take a small sample of a patient’s blood as a way of diagnosing early on or to establish the favorable response to future treatments.”
Issazadeh-Navikas also envisions the possibility of detection of damaged mitochondrial DNA in the bloodstream, making it feasible to diagnose the disease or gauge treatment responses through a simple blood test.
The researchers’ next endeavor involves investigating how mitochondrial DNA damage can serve as predictive markers for different disease stages and progression. “Furthermore, we are dedicated to exploring potential therapeutic strategies aimed at restoring normal mitochondrial function to rectify the mitochondrial dysfunctions implicated in the disease.”
You can read “Mitochondrial DNA damage triggers spread of Parkinson’s disease-like pathology” in Molecular Psychiatry.
More information:
Emilie Tresse et al, Mitochondrial DNA damage triggers spread of Parkinson’s disease-like pathology, Molecular Psychiatry (2023). DOI: 10.1038/s41380-023-02251-4
Journal information:
Molecular Psychiatry
Source: Read Full Article