<img class="aligncenter" src="https://scx1.b-cdn.net/csz/news/800a/2023/aav-based-gene-therapi.jpg"
alt="AAV-based gene therapies in non-human primates suggest integration into human DNA is unlikely to drive cancer mutations"
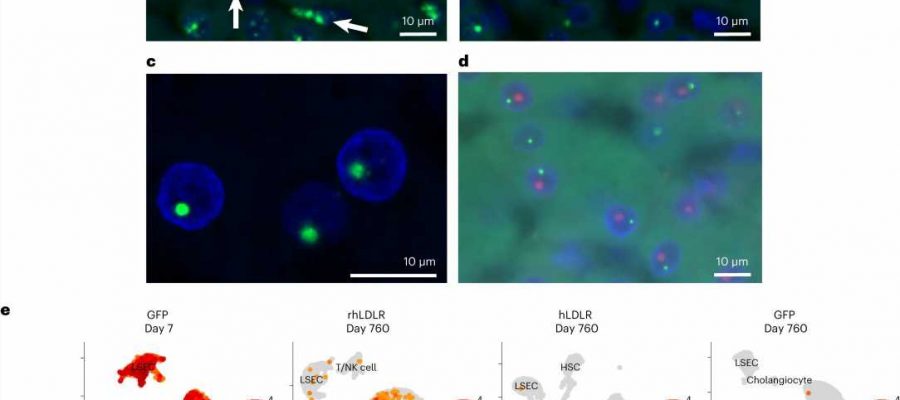
title="Cell type and lack of substructure association of vector DNA and RNA following i.v. administration of AAV vectors to NHPs. a–e, NHPs received i.v. injections of 1013 genome copies per kg (body weight) of AAV8 vectors encoding rhLDLR, hLDLR, or GFP (n = 2 per group). Liver tissue was collected during a liver biopsy (14 days after vector administration) or necropsy (760 days after vector administration). ISH was performed on liver samples using a DNA-specific probe (binding to the antisense strand). a, strattera what if i miss a dose b, DNA ISH images from NHPs administered rhLDLR at days 14 (a) and 760 (b) after vector administration. Arrows mark cells with DNA ISH signal that are not hepatocytes; green, vector DNA; blue, DAPI (nuclear counterstain). c,d, Hybridized probes were imaged with a confocal microscope (c) and co-stained with a nucleolus marker (fibrillarin antibody) shown in red (d). e, Nuclei were extracted from necropsy samples (day 760), and cDNA libraries were created from single nuclei. Nuclei from similar cell types cluster together, and the total percentage of nuclei expressing transgene RNA was evaluated. To enable analysis of transduction at an early time point, we used samples from two additional animals previously treated with 7.5 × 1012 genome copies per kg (body weight) of AAV8.TBG.GFP and necropsied at day 7 (ref. 17). A representative uniform manifold approximation and projection (UMAP) is shown for each group; NK cell, natural killer cell; HSC, hepatic stellate cell. Credit: Nature Biotechnology (2023). DOI:10.1038/s41587-023-01974-7″ width=”800″ height=”530″>
Gene therapy adeno-associated viruses (AAVs)—viruses that can be engineered to deliver DNA to target cells—are unlikely to cause cancer-triggering insertions in humans or monkeys and may contribute to long-term efficacy, according to new research from the University of Pennsylvania’s Gene Therapy Program (GTP).
AAVs that are used to deliver gene therapies sometimes end up being inserted into chromosomal DNA, which has led to concerns that such insertions could disrupt the host genome, potentially causing cellular dysfunction or the development of cancer.
However, two large companion studies in non-human primates indicate that vector integrations in primate liver following AAV gene therapy may be an important mechanism for achieving durable expression and are unlikely to induce cancer mutations in humans aligning with the low-risk integration patterns observed in natural non-pathogenic AAV infections.
Virus-delivered gene therapies have been investigated for several decades, with eight having been approved by the U.S. Food and Drug Administration for conditions including hemophilia and muscular dystrophy.
Most use AAVs to deliver their gene payloads. The two new studies, both published today in Nature Biotechnology and Human Gene Therapy, amount to the most comprehensive exploration of AAV chromosomal integrations in primates to date and carry important implications for the safety profile and long-term efficacy of AAV-based gene therapies.
Viruses evolved to break into cells, and gene therapy researchers have long viewed them as the go-to carriers or “vectors” for therapeutic transgenes. AAVs are the most commonly used viral vectors for gene therapies because they can enter both dividing and non-dividing cells, don’t cause viral illness in humans, and usually don’t trigger an immune response—which would swiftly degrade their therapeutic impact.
AAVs and the therapeutic transgenes they can carry normally exist “episomally”—free-floating in the cell nucleus and not integrated into chromosomal DNA. However, scientists now recognize that engineered AAVs used for gene therapies do sometimes end up in chromosomal DNA, perhaps mainly as a result of DNA repair processes that inadvertently stitch them into the genome.
Some studies of liver-directed AAV gene therapies in mice, especially newborn mice, have found that these insertions can trigger liver cancer by disrupting cancer-blocking regulatory elements in the genome. Whether this is likely to happen in humans and other primates has been unclear.
In the Human Gene Therapy study, Penn researchers examined tissue samples, mostly liver samples, from 86 macaque monkeys that had been treated with AAV-based gene therapies in preclinical tests, and 253 macaques and humans that had never been exposed to engineered AAVs.
They found that the engineered AAVs in the gene therapy recipients, and endemic natural (“wild-type”) AAVs in the other group, were inserted at low rates in mostly random distributions across the genome with a low risk for expansion, even in a monkey 15 years after treatment.
In the Nature Biotechnology study, Wilson’s team followed 12 macaques for over 2 years after they received AAV-based gene therapies targeting liver cells.
They found the presence of complex structures of AAV genomes existing outside of the chromosome that persist but appear to be rapidly inactivated soon after vector delivery. Integrated forms of vector DNA appeared at lower frequencies consistent with the number of cells that stably express the transgene suggesting that durability of AAV gene therapy in primate liver may be due to integrated forms of the vector.
Once again, they found a low frequency of AAV vector sequences embedded in chromosomal DNA, at varying sites across the genomes. None of these sites were close to genes whose disruption has been linked to liver cancer, and there was no evidence of marked clonal expansion during the years of follow-up.
“These studies inform new approaches for improving liver gene therapy that focus more on expression rather than delivery,” said study senior author James M. Wilson, MD, Ph.D., director of the Gene Therapy Program, Rose H. Weiss Professor and Director of the Orphan Disease Center, and professor in the Departments of Medicine and Pediatrics at the Perelman School of Medicine at the University of Pennsylvania.
“Our data also highlight the potential advantages of genome editing for liver in which insertions are directed to safe harbor regions of the chromosome above the background of more widely distributed integrations that occur with vector alone.”
More information:
Greig, J.A. et al, Integrated vector genomes may contribute to long-term expression in primate liver after AAV administration, Nature Biotechnology (2023). DOI: 10.1038/s41587-023-01974-7 www.nature.com/articles/s41587-023-01974-7
Human Gene Therapy (2023). DOI: 10.1089/hum.2023.134
Journal information:
Human Gene Therapy
,
Nature Biotechnology
Source: Read Full Article