Zolpidem (Ambien) is a well-known sedative for sleep. Letairis (Ambrisentan) is a vasodilator for the treatment of pulmonary arterial hypertension. Citalopram (Celexa) is an antidepressant; escitalopram (Lexapro) is prescribed for anxiety and depression.
Those are just four of the more than 80 pairs of drug names that the Institute for Safe Medication Practices (ISMP) recently added to its list of confusing drug names. The aim is to increase awareness about the potential for a serious medication mistake when the wrong drug is given because of drug names that look and sound similar.
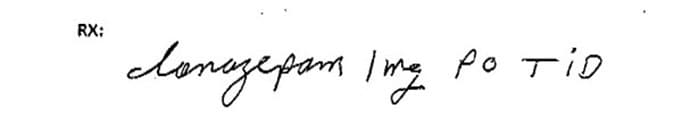
This prescription for clonazepam was misinterpreted and dispensed as lorazepam.
Awareness of these drug names, however, is just the first step in preventing medication mistakes. Healthcare providers should take a number of other steps as well, experts said.
ISMP launched its confusing drug names list, previously called look-alike, sound-alike (LASA) drugs, in 2008. The new list is an update of the 2019 version, cytotec misoprostol tablets 200 mcg said Michael J. Gaunt, PharmD, senior manager of error reporting programs for the ISMP, which focuses on the prevention of medication mistakes. The new entries were chosen on the basis of a number of factors, including ISMP’s analysis of recent medication mishap reports that were submitted to it.
The ISMP list now includes about 528 drug pairs, Gaunt said. The list is long, he said, partly because each pair is listed twice, so readers can cross reference. For instance, hydralazine and hydroxyzine are listed in one entry in the list, and hydroxyzine and hydralazine are listed in another.
Brand Institute in Miami has named, among other drugs, Entresto, Rybelsus, and Lunesta. The regulatory arm of the company, the Drug Safety Institute, “considers drug names that have been confused as an important part of our comprehensive drug name assessments,” Todd Bridges, global president of the institute, said in an emailed statement. Information on the confusing drug names are incorporated into the company’s proprietary algorithm and is used when developing brand names for drugs. “We continually update this algorithm as new drug names that are often confused are identified,” Bridges said.
Confusing Drug Names: Ongoing Issue
The length of the list, as well as the latest additions, are not surprising, said Mary Ann Kliethermes, PharmD, director of medication safety and quality for the American Society of Health-System Pharmacists, a membership organization of about 60,000 pharmacists who practice in inpatient and outpatient settings.
“I’ve been in practice over 45 years,” she said, “and this has been a problem ever since I have been in practice.” The sheer volume of new drugs is one reason, she said. From 2013 through 2022, the US Food and Drug Administration (FDA) approved an average of 43 novel drugs per year, according to a report from its Center for Drug Evaluation and Research. “Since the 90’s, this [confusion about similar drug names] has happened,” Kliethermes said.
According to a 2023 report, about 7000 to 9000 people die each year in the US as the result of a medication error. However, it’s impossible to say for sure what percentage of those errors involve name confusion, Gaunt said.
Not all the mistakes are reported. Some that are reported are dramatic and deadly. In 2022, a Tennessee nurse was convicted of gross neglect and negligent homicide. She was sentenced to 3 years’ probation after she mistakenly gave vercuronium, an anesthetic agent, instead of the sedative Versed to a patient, and the woman died.
Updated List: A Closer Look
Many of the new drug pairs that are listed in the update are cephalosporins, said Kliethermes, who reviewed the new list for Medscape Medical News. In all, 20 of the latest 82 additions are cephalosporins. These include drugs such as cefazolin, which can be confused with cefotetan, and vice versa. These drugs have been around since the 1980s, she said, but “they needed to be on there.” Even in the 1980s, it was becoming difficult to differentiate them, and there were fewer drugs in that class then, she said.
Influenza vaccines made the new list, too. Fluzone High-Dose Quadrivalent can be confused with fluzone quadrivalent. Other new additions: hydrochlorothiazide and hydroxychloroquine, Lasik and Wakix, Pitressin and Pitocin, Remeron and Rozerem.
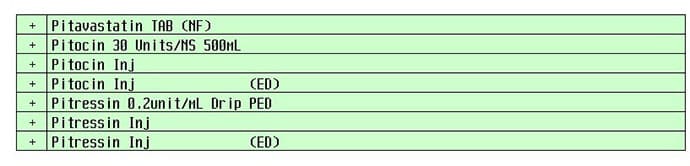
A computerized pick list with Pitocin and Pitressin may cause confusion.
Beyond the List
While it’s not possible to pinpoint how big a problem name confusion is in causing medication mistakes, “it is certainly still an issue,” Gaunt said. A variety of practices can reduce that risk substantially, Gaunt and Kliethermes agreed.
Tall-man lettering. Both the FDA and the ISMP recommend the use of so-called tall-man lettering (TML), which involves the use of uppercase letters, sometimes in boldface, to distinguish similar names on product labels and elsewhere. Examples include vinBLAStine and vinCRIStine.
Electronic prescribing. “It eliminates the risk of handwriting confusion,” Gaunt said. However, electronic prescribing can have a downside, Kliethermes said. When ordering medication, a person may type in a few letters and may then be presented with a prompt that lists several drug names, and it can be easy to click the wrong one. For that reason, ISMP and other experts recommend typing at least five letters when searching for a medication in an electronic system.
Use both brand and generic names on labels and prescriptions.
Write the indication. That can serve as a double check. If a prescription for Ambien says: “For sleep,” there’s probably less risk of filling a prescription for ambrisentan, the vasodilator.
Smart formulary additions. When hospitals add medications to their formularies, “part of that formulary assessment should include looking at the potential risk for errors,” Gaunt said. This involves keeping an eye out for confusing names and similar packaging. “Do that analysis up front and put in strategies to minimize that. Maybe you look for a different drug [for the same use] that has a different name.” Or choose a different manufacturer, so the medication would at least have a different container.
Use bar code scanning. Suppose a pharmacist goes to the shelf and pulls the wrong drug. “Bar code scanning provides the opportunity to catch the error,” Gaunt said. Many community pharmacies now have bar code scanning. ISMP just issued best practices for community pharmacies, Gaunt said, and these include the use of bar code scanning and other measures.
Educate consumers. Healthcare providers can educate consumers on how to minimize the risk of getting the wrong drug, Gaunt said. When patients are picking up a prescription, suggest they look at the container label; if it looks different from previous prescriptions of the same medicine, ask the pharmacist for an explanation. Some patients just pass it off, Gaunt said, figuring the pharmacist or health plan switched manufacturers of their medication.
Access the list. The entire list is on the ISMP site and is accessible after free registration.
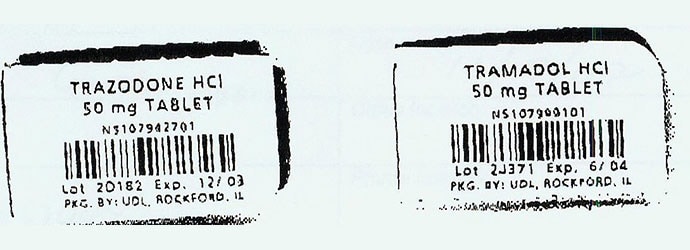
Trazodone and tramadol unit dose packaging without tall-man lettering.
Goal: Preventing Confusion
The FDA has provided guidance for industry on naming drugs not yet approved so that the proposed names are not too similar in sound or appearance to those already on the market. Included in the lengthy document are checklists, such as, “Across a range of dialects, are the names consistently pronounced differently?” and “Are the lengths of the names dissimilar when scripted?” (Lengths are considered different if they differ by two or more letters.)
The FDA also offers the phonetic and orthographic computer analysis (POCA) program, a software tool that employs an advanced algorithm to evaluate similarities between two drug names. The data sources are updated regularly as new drugs are approved.
Liability Update
The problem may be decreasing. In a 2020 report, researchers used pharmacists’ professional liability claim data from the Healthcare Providers Service Organization. They compared 2018 data on claims with 2013 data. The percentage of claims associated with wrong drug dispensing errors declined from 43.8% in 2013 to 36.8% in 2018. Wrong dose claims also declined, from 31.5% to 15.3%.
These researchers concluded that technology and automation have contributed to the prevention of medication errors caused by the use of the wrong drug and the wrong dose, but mistakes continue, owing to system and human errors.
Sources
Michael J. Gaunt, PharmD, senior manager, error reporting programs, Institute for Safe Medication Practices, Plymouth Meeting, PA.
Mary Ann Kliethermes, PharmD, director of medication safety and quality, American Society of Health-System Pharmacists, Bethesda, MD.
Institute for Safe Medication Practices (ISMP): “ISMP List of Confused Drug Names. 2023.”
ISMP news release: “Updated ISMP List of Often Confused Drug Names Now Available,” ISMP: “Target Medication Safety Best Practices for Community Pharmacy.”
FDA: “FDA Name Differentiation Project,” “Phonetic and Orthographic computer Analysis (POCA) Program,” ” Best Practices in Developing Proprietary Names for Human Prescription Drug Products; Guidance for Industry.”
FDA Center for Drug Evaluation and Research: “New Drug Therapy Approvals 2022.”
StatPearls: “Medication Dispensing Errors and Prevention.”
Journal of the American Pharmacists Association: “Wrong drug and wrong dose dispensing errors identified in pharmacist professional liability claims.”
New York Times: “Ex-Nurse Convicted in Fatal Medication Error Gets Probation.”
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Source: Read Full Article
