Several different vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been given emergency approvals in many countries after demonstrating their effectiveness in preventing severe disease and hospitalization due to the coronavirus disease 2019 (COVID-19) up to 20 weeks after vaccination.
 Study: SARS-CoV-2 Spike S1-specific IgG kinetic profiles following mRNA- versus vector-based vaccination in the general Dutch population. Image Credit: angellodeco / Shutterstock.com
Study: SARS-CoV-2 Spike S1-specific IgG kinetic profiles following mRNA- versus vector-based vaccination in the general Dutch population. Image Credit: angellodeco / Shutterstock.com
In the Netherlands, four vaccines have been included in the national vaccination program, buy norvasc no prescription h two of which are based on the messenger ribonucleic acid (mRNA) technology (Comirnaty and Spikevax), whereas the other two are vector-based (Vaxzevria and Janssen).
Notably, mRNA-based vaccines use lipid nanoparticles to deliver spike-encoding mRNA, whereas vector-based vaccines use adenovirus to deliver spike-encoding deoxyribonucleic acid (DNA) to induce the production of neutralizing antibodies against the viral spike protein by host cells.
Multiple reports have independently described the induction of spike-specific antibodies by current COVID-19 vaccines. However, most of these results were obtained from specific groups, like the immunocompromised individuals or healthcare workers. As a result, the direct comparisons of mRNA- and vector-based vaccines in the general population are scarce, as are those comparing these four vaccines simultaneously.
The PIENTER-Corona (PiCo) study is a Dutch nationwide efficacy comparison study, wherein participants 18 years or older who have received one or two doses of Comirnaty, Spikevax, Vaxzevria, or Janssen are being compared for SARS-CoV-2 spike S1-specific (S1) antibody concentrations induced by each vaccine type. A recent report on the initial results of the PiCo study has been published on the preprint server medRxiv*.
About the study
In the current study, the researchers compared (S1) immunoglobulin G (IgG) antibodies post-vaccination with mRNA-based (Comirnaty, Spikevax) or vector-based (Janssen, Vaxzevria) vaccines using samples from a Dutch nationwide cohort.
The data was collected in February and June 2021 following the launch of the Dutch national COVID-19 vaccination campaign from public health databases. Data availability was driven by vaccine rollout and the prioritization of the aging population during the campaign.
For those between the ages of 18 to 64, data were available up to two months following each dose across all four vaccines for an overall total of 2,412 people. For the Comirnaty vaccine, data across all ages were available in SARS-CoV-2-naïve people between 18-91 years up to four months following the second dose for 196 people. S1 antibody concentrations were measured and expressed in binding antibody units per mL (BAU/mL)
The median vaccination interval between the two doses was 77 days for Vaxzevria, 35 days and 33 days both for Comirnaty and Spikevax. For infection-naïve adults between the ages of 18-64 years, mRNA-based vaccines induced S1 IgG faster and reached higher levels than vector-based vaccines. Subsequent to the rise in antibody concentrations following the second dose, an initial rapid decay could be seen until it eventually stabilized.
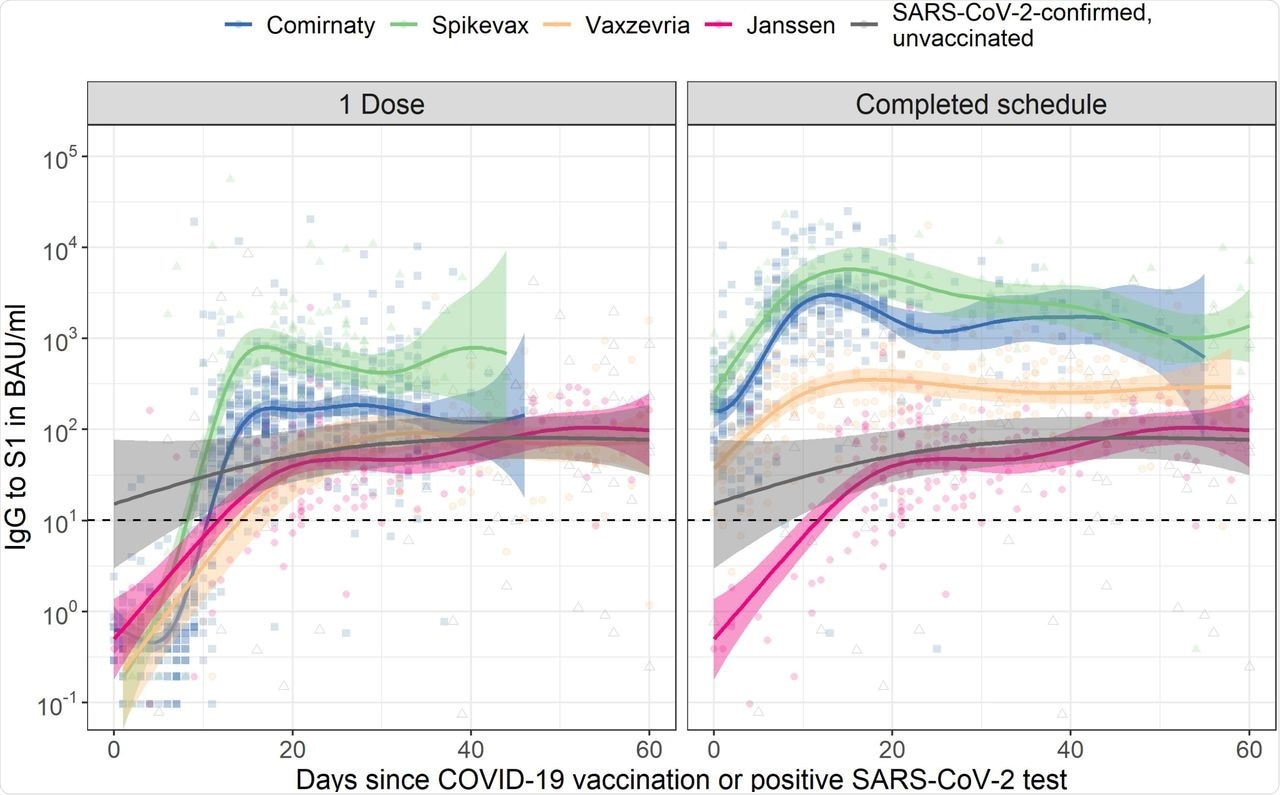 Spike S1 immunoglobulin G (IgG) kinetics following COVID-19 vaccination by number of doses and vaccine brand in SARS-CoV-2-naive adults aged 18 to 64 years old. The dashed horizontal line represents the threshold for seropositivity. Data for Janssen is duplicated across the two panels to enable direct comparison with the other vaccine brands after one dose and a completed vaccination schedule. For comparison, IgG concentrations following a positive SARS-CoV-2 PCR or antigen test in unvaccinated participants are shown alongside vaccination responses; data is duplicated in both panels (for details see Supplementary Table 3). Fit and 95% confidence bands are shown from a Generalized Additive Model, using penalized splines, with only time since dose in days as explanatory variable. For results of multivariable models, see Supplementary Table 1. BAU/mL: binding antibody units per mL; IgG: immunoglobulin G.
Spike S1 immunoglobulin G (IgG) kinetics following COVID-19 vaccination by number of doses and vaccine brand in SARS-CoV-2-naive adults aged 18 to 64 years old. The dashed horizontal line represents the threshold for seropositivity. Data for Janssen is duplicated across the two panels to enable direct comparison with the other vaccine brands after one dose and a completed vaccination schedule. For comparison, IgG concentrations following a positive SARS-CoV-2 PCR or antigen test in unvaccinated participants are shown alongside vaccination responses; data is duplicated in both panels (for details see Supplementary Table 3). Fit and 95% confidence bands are shown from a Generalized Additive Model, using penalized splines, with only time since dose in days as explanatory variable. For results of multivariable models, see Supplementary Table 1. BAU/mL: binding antibody units per mL; IgG: immunoglobulin G.
For vector-based vaccines, the slower rise in S1 IgG levels stabilized without any clear decay. Between fourteen days and two months after completion of the vaccination schedule or positive SARS-CoV-2 test, median IgG levels were 2,799 BAU/mL for Spikevax, 2,408 BAU/mL for Comirnaty, 313 BAU/mL for Vaxzevria, 64 BAU/mL for Janssen, and 91 BAU/mL for unvaccinated SARS-CoV-2-confirmed participants.
All three non-responders for Spikevax, Comirnaty, and Vaxzevria had a high risk for comorbidities. In the Janssen recipients, S1 IgG levels increased to 77 BAU/mL with no comorbidity for the two non-responders twenty-eight days after vaccination. Regression results showed that age, sex, and the presence of comorbidities significantly contributed to S1 IgG concentrations; however, this was inconsistent between vaccines and doses.
In adults aged 18-64, S1 IgG concentrations were higher in people with a history of SARS-CoV-2 after the first dose as compared to previously naïve people, irrespective of vaccine type. For people with a history of SARS-CoV-2 infection, no further increase occurred after a second dose, if applicable.
For the Comirnaty vaccine, IgG levels were more heterogeneous in the oldest age group. However, these levels were not significantly different between infection-naïve adults aged 18-64 years (436 BAU/mL) and those aged 65-79 (542 BAU/mL) and ≥80 years old (366 BAU/mL).
Implications
The current study provided highly relevant confirmation of results from controlled vaccine trials showing high immunogenicity post-vaccination in the general population, including vulnerable groups and different vaccination regimens.
Although most people seroconverted, irrespective of the vaccine received, mRNA- and vector-based COVID-19 vaccines induced distinct S1-specific IgG kinetic profiles, with mRNA vaccines inducing higher concentrations among the general population. Further studies should be conducted to explore the protection conferred against severe infection, as well as the levels of cell-mediated immunity induced by different vaccines.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- van den Hoogen, L.L., Verheul, M.K., Vos, E. R. A., et al. (2021). SARS-CoV-2 Spike S1-specific IgG kinetic profiles following mRNA- versus vector-based vaccination in the general Dutch population. medRxiv. doi:10.1101/2021.10.25.21265467. https://www.medrxiv.org/content/10.1101/2021.10.25.21265467v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Adenovirus, Aging, Antibodies, Antibody, Antigen, Cell, Coronavirus, Coronavirus Disease COVID-19, DNA, Efficacy, Healthcare, immunity, Immunoglobulin, Nanoparticles, Protein, Public Health, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine

Written by
Sreetama Dutt
Sreetama Dutt has completed her B.Tech. in Biotechnology from SRM University in Chennai, India and holds an M.Sc. in Medical Microbiology from the University of Manchester, UK. Initially decided upon building her career in laboratory-based research, medical writing and communications happened to catch her when she least expected it. Of course, nothing is a coincidence.
Source: Read Full Article
