The findings of a clinical trial by Trinity College Dublin researchers of treatment for atopic dermatitis have been published today in The Lancet journal (Friday, 21st May, 2021). Results of the clinical trial at the School of Medicine, Trinity College and St James’s Hospital, Dublin have shown the drug upadacitinib to be the most effective treatment to date for this chronic, relapsing inflammatory condition. The research is vital as there is an unmet need which exists for therapies that provide remission of symptoms in moderate-to-severe atopic dermatitis.
The publication reports efficacy and safety results of upadacitinib compared with placebo for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents. This pivotal Global Phase 3 study involved 1,600 patients and took place over the last two years at The Wellcome Trust/Health Research Board Clinical Research Facility at St James’s Hospital.
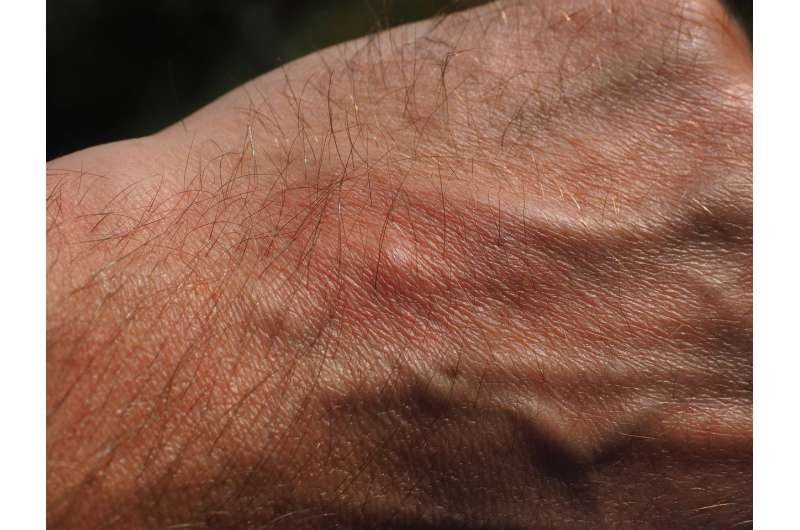
Atopic dermatitis is a chronic, relapsing inflammatory condition characterized by a cycle of intense itching and scratching leading to cracked, scaly, oozing skin. It affects up to an estimated 10 percent of adults and 25 percent of children. Between 20 and 46 percent of adults with atopic dermatitis have moderate to severe disease. The range of symptoms pose significant physical, psychological and economic burden on individuals impacted by the disease.
Results show upadacitinib to so far be the most effective treatment for atopic dermatitis in clinical trials. The magnitude and breadth of the treatment effect versus placebo across multifaceted aspects of atopic dermatitis provides evidence that a targeted therapy blocking multiple inflammatory pathways could help to address the substantial unmet needs in the treatment of moderate-to-severe atopic dermatitis.
These pivotal findings could potentially transform the treatment goals and standards of care for patients with moderate-to-severe atopic dermatitis.
Professor Alan Irvine, School of Medicine, Trinity College and Principal Investigator said:
Source: Read Full Article
