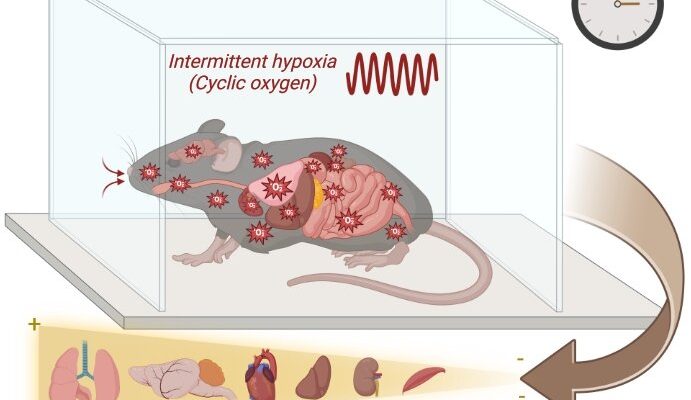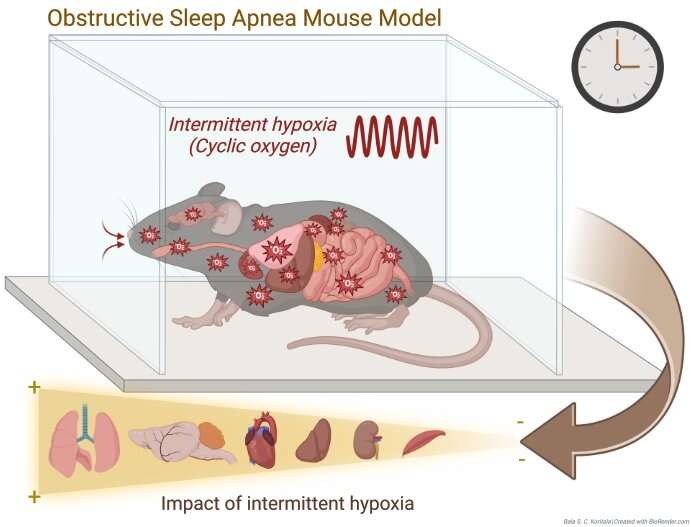
The low blood oxygen levels of obstructive sleep apnea (OSA) cause widespread changes in gene activity throughout the day, according to a new study in the open-access journal PLOS Biology by David Smith of Cincinnati Children’s Hospital Medical Center, US, and colleagues. The finding may lead to tools for earlier diagnosis and tracking of the disorder.
OSA occurs when the airway becomes blocked (usually by soft tissue, associated with snoring and interrupted breathing during the night), resulting in intermittent hypoxia (low blood oxygen) and disrupted sleep. It affects over one billion people worldwide and costs $150 billion per year in direct medical costs in the United States alone. OSA increases the risk for cardiovascular, respiratory, metabolic, and neurologic complications.
The activity of many genes varies naturally throughout the day, partially in response to activity of circadian clock genes, whose regular oscillations drive circadian variation in up to half the genome. Gene activity also varies in response to external factors, including decreases in oxygen levels, which causes production of “hypoxia-inducible factors,” which influence activity of many genes, including clock genes.
To better understand how OSA may affect gene activity throughout the day, the authors exposed mice to intermittent hypoxic conditions and examined whole-genome transcription in six tissues—lung, liver, kidney, muscle, heart, and cerebellum—throughout the day. The authors then evaluated variation in the circadian timing of gene expression in these same tissues.
The largest changes were found in lung, where intermittent hypoxia affected transcription of almost 16% of all genes, most of which were upregulated. Just under 5% of genes were affected in heart, liver, and cerebellum.
The subset of genes that normally exhibit circadian rhythmicity were even more strongly affected by intermittent hypoxia, with significant changes seen in 74% of such genes in the lung and 66.9% of such genes in the heart. Among the genes affected in each tissue were known clock genes, an effect that likely contributed to the large changes in circadian activity of other genes seen in these tissues.
“Our findings provide novel insight into the pathophysiological mechanisms that could be associated with end-organ damage in patients with chronic exposure to intermittent hypoxia,” Smith said, “and may be useful to identify targets for future mechanistic studies evaluating diagnostic or therapeutic approaches;” for instance, through a blood test tracking one of the dysregulated gene products to detect early OSA.
Bala S. C. Koritala adds, “Our study using an animal model of obstructive sleep apnea unveils time- and tissue-specific variations of the whole genome transcriptome and associated hallmark pathways. These unique findings uncover early biological changes linked to this disorder, occurring across multiple organ systems.”
More information:
Koritala BSC, Lee YY, Gaspar LS, Bhadri SS, Su W, Wu G, et al. (2023) Obstructive sleep apnea in a mouse model is associated with tissue-specific transcriptomic changes in circadian rhythmicity and mean 24-hour gene expression, PLoS Biology (2023). DOI: 10.1371/journal.pbio.3002139
Journal information:
PLoS Biology
Source: Read Full Article