In Munich, Germany, scientists at Ludwig Maximilian University studied temporal adaptive immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to better understand protection against re-infection. They found functional properties of persisting memory B specific to SARS-CoV-2 may help in protect against the virus.
When a pathogen infects, it elicits an active immune response. The adaptive immunity protects the individual against re-infection. It is associated with the development of antibodies, memory B cells, and several T cell subsets.
Analysis of immune responses to viruses, including coronaviruses, has shown that the lifespan of adaptive immunity varies. Even antibody levels decrease with time. It is reported that the serum antibodies against SARS-CoV-2 persist for more than 6 months after primary infection.
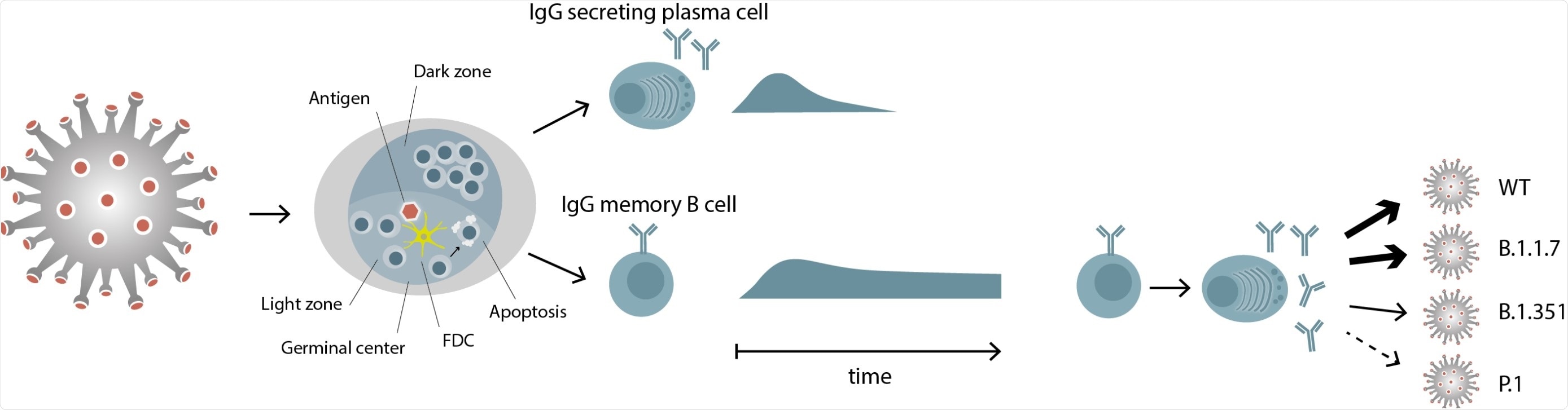
However, in case of mild infection, it is also found that these patients rapidly lose their specific antibodies. While the initial antibody responses are from short-lived plasmablasts (a short-lived differentiation stage between a post germinal center B-cell and a mature plasma cell), the subsequent development of high-affinity and persistent antibodies come from affinity maturation and expansion of B cells in the germinal centers.
Thus, it is vital to study the memory B cell pool, in addition to antibody titers, to estimate the humoral immunity as an indicator of immune protection. In their recent medRxiv* preprint research paper, Prof. Edgar Meinl et al. analyzed the persistence of Immunoglobulin A (IgA) and Immunoglobulin G (IgG) memory B cells specific for SARS-CoV-2 in COVID-19 patients.
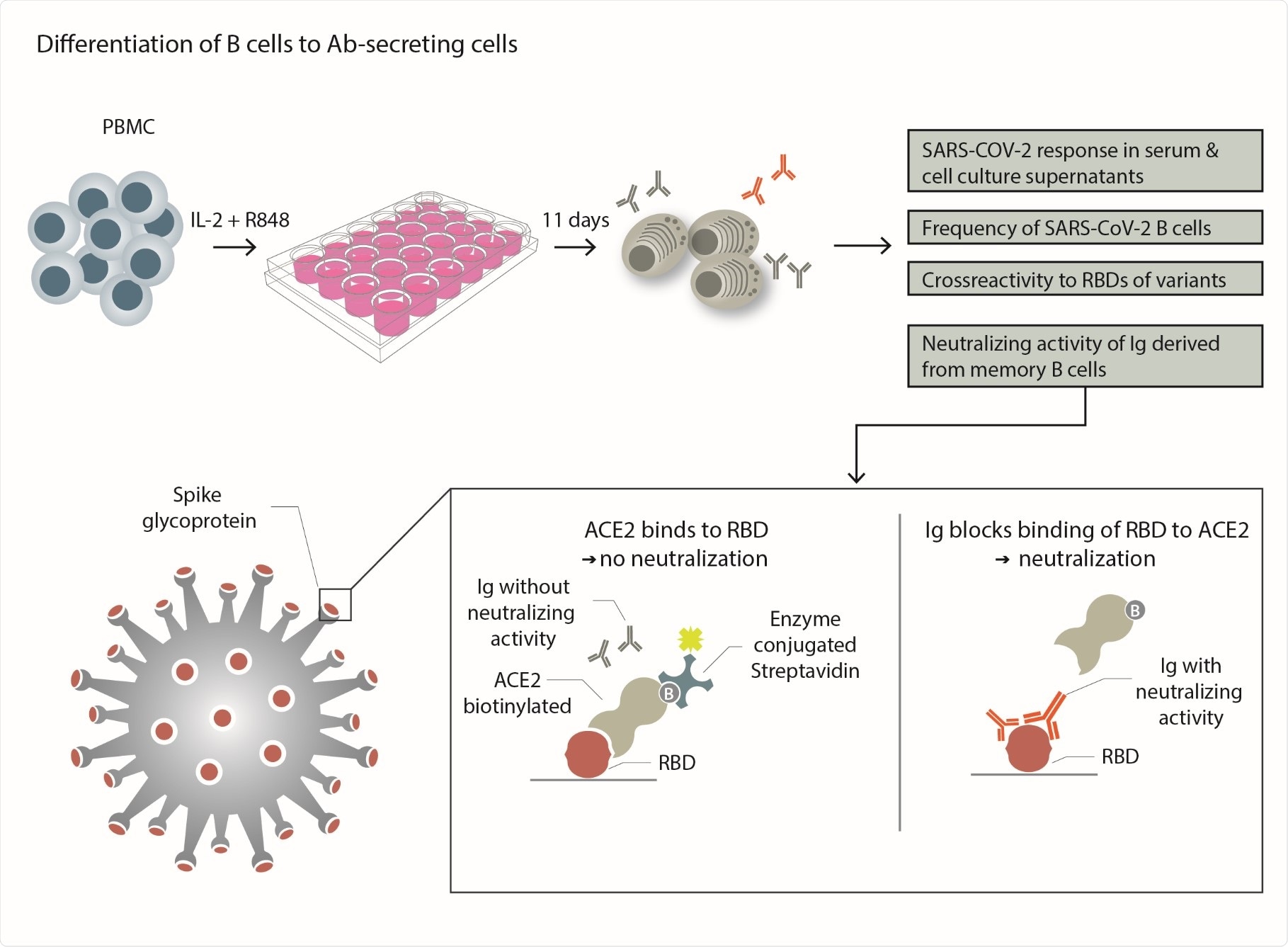
The researchers specifically looked for SARS-COV-2 infected and recovered individuals who had lost circulating IgG specific to SARS-CoV-2. The researchers investigated if these individuals still harbored specific memory B cells in their blood.
"Memory-B-cell differentiation into antibody-producing cells is a more sensitive method for detecting the previous infection than measuring serum antibodies."
Significantly, one of the study's implications is that the assay employed to differentiate the B cells into antibody-producing cells in vitro is a more sensitive method for detecting previous infections than measuring the serum levels of SARS-CoV-2 IgG.
Specifically, the assay identifies if a seronegative individual was infected with SARS-CoV-2. "This is of relevance in epidemiology and for optimizing urgently needed immunosuppressive treatments," the researchers said.
This study detected circulating IgG memory B cells specific for SARS-CoV-2 in all 16 patients, about 1–8 months after infection. These patients underwent a mild or asymptomatic COVID-19 disease course.
Of these, they also found that 11 participants had specific IgA B cells. The researchers reported that the COVID-19 patients had significantly more SARS-CoV-2-specific IgA B cells than the healthy donors.
Notably, four patients who had lost specific serum IgG after 5–8 months had SARS-CoV-2-specific-B-cell levels which were comparable to those of seropositive donors.
"This study helps us to understand and start to develop models for predicting long-term protection against SARS-CoV-2."
To study the B cells, they converted the blood-derived B cells into antibody-secreting cells in vitro, and they analyzed the secreted antibodies for neutralizing activity against SARS-CoV-2. They found that the Immunoglobulins produced after in vitro differentiation blocked the receptor-binding domain (RBD) binding to the cellular receptor ACE-2 (angiotensin-converting enzyme), indicating high neutralizing activity.
Towards a potential therapeutic strategy, the researchers recommend, "These persisting B cells can be harnessed to identify a previous infection."
They also tested for cross-reactivity to the recently emerged SARS-CoV-2 variants of concern (VOCs) B.1.1.7, B.1.351, and P.1. The researchers observed that the memory-B-cell-derived IgGs recognized the RBD of variant B.1.1.7 (similarly to the wild-type). At the same time, reactivity to B.1.351 and P.1. decreased by 30% and 50%, respectively. The reduced activity in the latter cases calls for attention and a need for follow-up vaccinations.
Although the recovered individual lost the specific circulating immunoglobulins, the presence of these B cells with neutralizing activity suggests that protection against possible re-exposure with the variant is possible.
"Taken together, the picture is emerging from this study and previous work that when circulating IgG and IgA, mucosal IgA, and circulating IgA memory B cells are gone, circulating IgG memory B cells persist."
This is an important study to understand the long-term protection against SARS-CoV-2. It focuses on the features of SARS-CoV-2-specific memory B cells: 1) circulating SARS-CoV-2 IgG memory B cells persist even after 6 months after infection, despite the loss of systemic IgG; 2) these cells produce neutralizing antibodies; 3) these immunoglobulins show differential cross-reactivity to emerging variants of concern.
This study raises the need to analyze if the patients become reinfected because they have lost a certain type of anti-SARS-CoV-2 immunity or because they are confronted with a new variant, against which they do not yet have a protective immunity, the researchers write.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Persistence of functional memory B cells recognizing SARS-CoV-2 variants despite loss of specific IgG, Stephan Winklmeier, Katharina Eisenhut, Damla Taskin, Heike Rübsamen, Celine Schneider, Peter Eichhorn, Oliver T. Keppler, Matthias Klein, Simone Mader, Tania Kümpfel, Edgar Meinl, medRxiv 2021.05.15.21257210; doi: https://doi.org/10.1101/2021.05.15.21257210, https://www.medrxiv.org/content/10.1101/2021.05.15.21257210v1
Posted in: Medical Research News | Disease/Infection News
Tags: Angiotensin, Antibodies, Antibody, Antigen, Assay, Blood, Cell, Coronavirus, Coronavirus Disease COVID-19, Enzyme, Epidemiology, Frequency, Immune Response, Immunoglobulin, in vitro, Lymph Nodes, Pathogen, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Virus

Written by
Dr. Ramya Dwivedi
Ramya has a Ph.D. in Biotechnology from the National Chemical Laboratories (CSIR-NCL), in Pune. Her work consisted of functionalizing nanoparticles with different molecules of biological interest, studying the reaction system and establishing useful applications.
Source: Read Full Article
