Immune cells play a key role in mediating inflammatory responses. Dysregulation in signaling mechanisms that operate across immune cells can trigger chronic inflammatory diseases like rheumatoid arthritis that cause pain and swelling in joints. One such immune cell known to be involved in autoimmune diseases is the dendritic cell. The activity of dendritic cells is regulated by the dendritic cell immunoreceptor (DCIR) present on their surface, which comprises a carbohydrate recognition domain that can bind to sugar moieties present on other proteins or cell surfaces, in a calcium dependent manner. The activity of osteoclast, which is involved in bone degradation, is also regulated by DCIR. However, little is known about the interacting partners of DCIR that help mediate inflammatory responses.
A team of researchers from Japan led by Professor Yoichiro Iwakura of the Department of Experimental Animal Science at the Research Institute for Biomedical Sciences, Tokyo University of Science, have now delved deeper into understanding the mechanisms underlying DCIR activity. In their previous work, the researchers reported that mice deficient in DCIR spontaneously develop arthritis and metabolic bone disorders.
Building on this finding, in a recent study published in the Journal of Experimental Medicine, they sought to elucidate DCIR binding partners and immune signaling mechanisms involved in inflammatory diseases. “In this study, we have identified a novel functional ligand of DCIR, likely involved in the pathogenesis of arthritis and other autoimmune diseases like multiple sclerosis. We are hopeful that our work can advance the research of immunology and glycobiology in inflammatory diseases,” explains Prof. Iwakura.
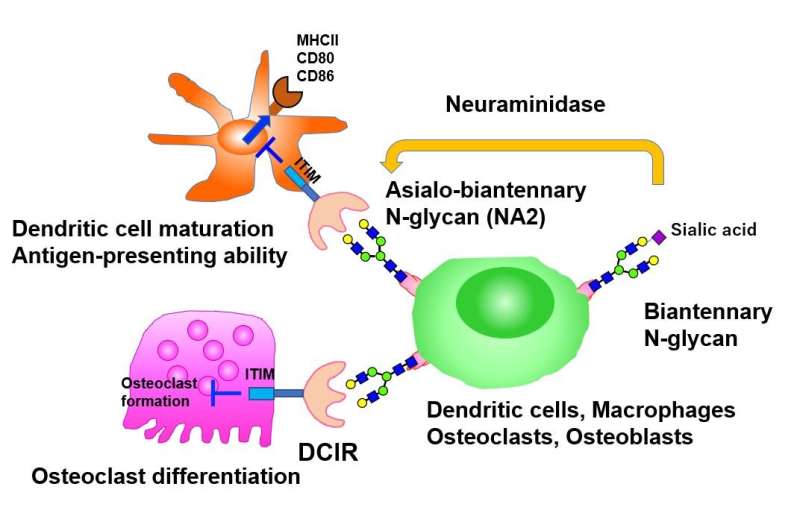
The researchers began by identifying potential ligands (molecules that bind to cell receptors) of DCIR on immune and bone cells, and found that DCIR binds to glycoproteins present on the surface of macrophages and osteoclasts, the latter differentiating from bone marrow derived macrophages (BMMs), and involved in bone degeneration and remodeling. On further characterization of the glycoprotein, they noted that this interaction was specific to asialo-biantennary N-glycan (NA2), a complex carbohydrate moiety comprising various sugar molecules.
Having identified the DCIR ligand, the team next sought to understand the effect of DCIR on osteoclast differentiation and osteoclastogenesis, a process contributing to bone loss. Interestingly, cells deficient in DCIR showed a significant increase in the expression of osteoclastogenesis associated genes. As the researchers speculated, expression of DCIR significantly suppressed the differentiation of osteoclasts, thus suggesting DCIR as an independent inhibitor of osteoclastogenesis. Further confirming this finding, a mutant version of DCIR, incapable of recognizing carbohydrate residues, was shown to not exhibit this inhibitory effect.
The role of DCIR in osteoclast differentiation, and its interacting ligand, NA2, now decoded, the team next examined the effect of NA2 on osteoclastogenesis. Consistent with their previous finding, NA2 treatment suppressed the differentiation of osteoclasts from wild-type BMMs but not from cells deficient in DCIR, underscoring the role of NA2 as a specific functional ligand of DCIR that suppresses osteoclastogenesis.
Taking a step further, the researchers treated mouse autoimmune disease models with neuraminidase, an enzyme that removes terminal sialic acid residues from N-glycan, thereby enhancing the exposure of NA2. Much to their delight, neuraminidase treatment further suppressed autoimmune diseases like autoimmune arthritis or experimental autoimmune encephalomyelitis, yet again, in a DCIR dependent manner! Furthermore, neuraminidase treatment ameliorated inflammation and associated bone loss in a mouse model of arthritis, thereby confirming their findings in vitro and in vivo. The inhibitory effect of DCIR-NA2 interaction on autoimmune diseases was found to be mediated through suppression of the antigen presenting ability of dendritic cells and subsequent decrease in the activation of other immune cells that contribute to inflammation.
Source: Read Full Article
