In a recent study posted to the medRxiv* preprint server, researchers University of Washington, University of Michigan, Duke University, and Veterans Affairs Portland Healthcare System assessed the effectiveness of the coronavirus disease 2019 (COVID-19) booster vaccines.
Various studies have reported that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant is more likely to evade the immune system and cause breakthrough COVID-19 infections than the other variants. In order to determine the effectiveness of booster vaccines in at-risk populations of Omicron, further research is needed.
 Study: Effectiveness of mRNA COVID-19 vaccine boosters against infection, hospitalization and death: a target trial emulation in the omicron (B.1.1.529) variant era. Image Credit: Davide Bonaldo / Shutterstock
Study: Effectiveness of mRNA COVID-19 vaccine boosters against infection, hospitalization and death: a target trial emulation in the omicron (B.1.1.529) variant era. Image Credit: Davide Bonaldo / Shutterstock
About the study
The present study investigated the messenger ribonucleic acid (mRNA) booster vaccine effectiveness (VE) against the SARS-CoV-2 Omicron infection and related hospitalization and death.
The team designed a retrospective matched cohort study to conduct a controlled trial of booster mRNA vaccination with the mRNA-1273 and BNT162n2 vaccines. The enrollment and follow-up for the study were between 1 December 2021 and 31 March 2022. The team employed a matched cohort design comprising eligible individuals who were matched based on their dates of booster vaccination to persons who were not boosted as of that date. The follow-up period for each participant lasted until the first occurrence of either an outcome event, death not causally related to COVID-19, booster vaccination of the comparator, or the end of the study period.
The team identified all Veterans Affairs (VA) enrollees who were aged 18 years and above, were alive as of 1 December 2021, and were vaccinated with two mRNA vaccine doses with the second vaccination received at least five months before the study. Almost 30% of the eligible participants had received their booster mRNA vaccine during the study period between 1 December 2021 and 31 March 2022.
The cohorts were matched every week of the study period by identifying eligible participants who were alive, not infected by SARS-CoV-2, and had not yet been boosted. Among these persons, the team selected potential individuals to be included in the treatment arm, which comprised persons who received a booster dose during that week and comparators who were not boosted. Baseline characteristics were used to recognize the best-matched comparators corresponding to each boosted participant. The exact matching was performed based on age, Charlson comorbidity index (CCI), type of primary COVID-19 vaccine received, and the date when the primary vaccination was completed.
The primary end-points of the study included COVID-19 infection, related hospitalization, and death. The team defined SARS-CoV-2 infection as a positive result for the SARS-CoV-2 antigen in a respiratory sample or nucleic acid amplification test. The date of the infection was considered to be the earliest date of SARS-CoV-2 positive diagnosis. SARS-CoV-2-related hospitalization was defined as hospital admission recorded in the VA system or within 30 days of a SARS-CoV-2 positive test and related death.
Results
The study results showed that almost 99% of the matched cohorts were under follow-up and considered to be at-risk at day 10 since the time of positive SARS-CoV-2 diagnosis. While the average follow-up duration was approximately 79.8 days for both the matched cohorts, a total of 6438 and 10,946 SARS-CoV-2 infections were detected in the booster and the no-booster groups, respectively. The team noted that 85% of these COVID-19 infections occurred after 1 January 2022. Moreover, almost 100%, 14%, and 0.5% of the infections detected after 1 January 2022, between 16 December and 31 December 2022, and before 16 December 2022 were caused by the SARS-CoV-2 Omicron variant, respectively.
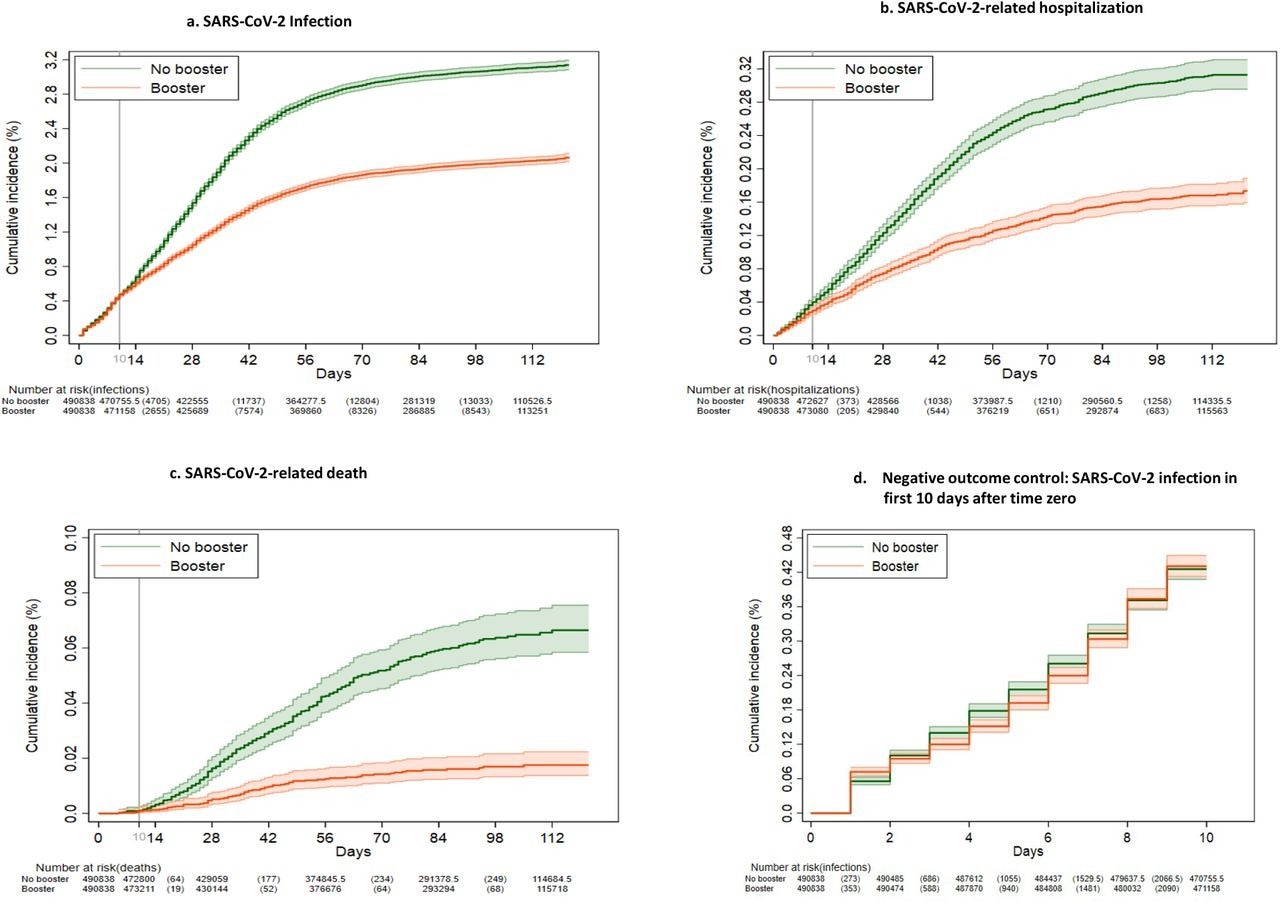
Kaplan-Meier curves comparing persons who received a booster mRNA COVID-19 vaccine versus their matched counterparts who did not with respect to the cumulative incidence (%) and 95% confidence intervals of SARS-CoV-2 infection (a), SARS-CoV-2-related hospitalization (b), SARS-CoV-2-related death (c) and SARS-CoV-2 infection during the first 10 days after time zero (d) (negative outcome control).
The team also observed that overall booster VE against Omicron infections was 42.3%, while that for the BNT162b2 booster was 39.0% and the mRNA-1273 booster was 44.6%. Furthermore, the VE of the booster vaccines was higher among persons who had received their primary vaccination more than nine months before the booster as compared to individuals who were vaccinated five to nine months prior. Booster VE was also found to be higher among individuals reporting more comorbidities but lower among the elderly population.
A total of 515 deaths were observed in the booster group while 1060 deaths were found in the no-booster cohort. The team also remarked that the cumulative incidence of hospital admission and death due to COVID-19 at 121 days was significantly lower among the boosted persons than the non-boosted individuals. The VE of booster vaccines against hospitalization and death was 53.3% and 79.1% overall and 54% and 85.5% for the BNT162b2 and 52.9% and 75.2% for the mRNA-1273 booster cohorts, respectively. Moreover, VE was higher among individuals who were boosted more than nine months after primary vaccination and persons having a higher number of comorbidities.
Overall, the study findings showed that the mRNA booster vaccines displayed sufficient VE against SARS-CoV-2 infection, related hospitalization, and death. Therefore, the researchers believe that booster vaccination should be encouraged to reduce the number of deaths related to COVID-19.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Effectiveness of mRNA COVID-19 vaccine boosters against infection, hospitalization and death: a target trial emulation in the omicron (B.1.1.529) variant era, George N. Ioannou, Amy S.B. Bohnert, Ann M. O'Hare, Edward J. Boyko, Valerie A. Smith, Matthew L. Maciejewski, C. Barrett Bowling, Elizabeth Viglianti, Theodore J. Iwashyna, Denise M. Hynes, Kristin Berry, COVID-19 Observational Research Collaboratory (CORC), medRxiv, 2022.06.15.22276466, DOI: https://doi.org/10.1101/2022.06.15.22276466, https://www.medrxiv.org/content/10.1101/2022.06.15.22276466v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antigen, Coronavirus, Coronavirus Disease COVID-19, Healthcare, Hospital, Immune System, Nucleic Acid, Omicron, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Veterans Affairs

Written by
Bhavana Kunkalikar
Bhavana Kunkalikar is a medical writer based in Goa, India. Her academic background is in Pharmaceutical sciences and she holds a Bachelor's degree in Pharmacy. Her educational background allowed her to foster an interest in anatomical and physiological sciences. Her college project work based on ‘The manifestations and causes of sickle cell anemia’ formed the stepping stone to a life-long fascination with human pathophysiology.
Source: Read Full Article
