A new multidisciplinary study conducted by researchers at the University of Rochester Medical Center has found that the co-development of three systems, the gut microbiome, respiratory system and immune system, is correlated with a baby’s respiratory health, and an infant can have negative respiratory outcomes if the development of one of these systems is disrupted.
The study, “Aberrant newborn T cell and microbiota developmental trajectories predict respiratory compromise during infancy,” was published in iScience and was co-authored by Kristin Scheible, M.D., associate professor in the departments of Pediatrics and Microbiology and Immunology, and Andrew McDavid, Ph.D., from the department of Biostatistics and Computational Biology.
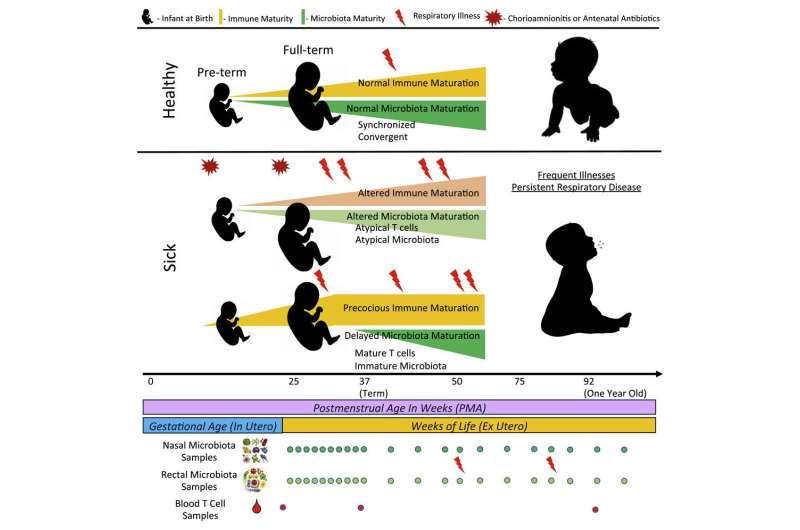
The project looked at 148 preterm and 119 full-term infants from birth through one year of age to examine the development of the microbiome, immune, and respiratory systems and how this co-development affects the health of a child. These systems typically develop concurrently and in sync with the baby during its first year of life.
The study found that disruption of any of the three systems resulted in greater respiratory morbidity for infants. In addition, the postmenstrual age (or weeks from conception) of the baby was a more accurate benchmark for predicting disruption of any of the systems than time since birth.
“When a baby is born, that is typically considered day zero for that child. We instead modeled it with the baby’s age starting at the day of conception,” said Scheible. “A baby’s immune and microbial development at two months old doesn’t look the same for a baby born at 32 weeks compared to one born at 42.”
The implications of using postmenstrual age as a benchmark could potentially change how clinicians view the risk and benefit of immune or microbial-altering therapies, such as antibiotics or probiotics. This study found that prenatal antibiotics or infection disrupts the developmental trajectory. If babies are exposed to antibiotics—especially premature infants—that increases their risk for respiratory illnesses in the first year of life.
Additionally, caregivers should examine the usage of pro and prebiotics, according to Scheible. These interventions may not work when introduced at an inappropriate development timeline, and clinicians should consider using the postmenstrual age as a measure of readiness to see benefit from therapies targeting the microbiome and immune systems.
“When you take a baby that is born premature and strip away all the protections of the mother, such as the placenta, it’s critical to know what happens to underdeveloped systems like the microbiome and the immune system. Interventions like intubation, central lines, oxygen and antibiotics are implemented and influence their development, and the impact of perturbing these systems may ripple more widely in those first two critical weeks for the baby,” said Scheible.
To this point, the study found that when the fetus is exposed to antibiotics or infection right before birth, the normal T cell population developmental trajectory is disrupted, and this disruption predicts subsequent respiratory microbial colonization and respiratory illness. In addition, the study authors found than when either the microbiome, immune, or respiratory system is disrupted, the three systems are no longer on a parallel path of development, and it takes several years for the affected system to catch up.
“We were able to model and measure immune and microbiome development and compare it with the clinical history of the patient, and the asynchrony of these systems directly leads to worse respiratory outcomes,” said McDavid.
More research will be needed to validate these findings and to pin down the mechanisms linking microbial-immune co-development. If confirmed, these results could have major implications for determining risk/benefits of perinatal antibiotic administration, the optimal timing for immune and microbiota targeting interventions, and for predicting potential respiratory morbidity for exposed pre- and full-term infants.
Source: Read Full Article
