Antibodies that can disarm a virus, known as neutralizing antibodies, are key to the body’s ability to fight off infection. MIT chemists have come up with a new way to identify these neutralizing antibodies in a blood sample, by analyzing how antibodies interact with sugar molecules found on the surface of a viral protein.
The new test could help to reveal whether someone has neutralizing antibodies against viruses such as SARS-CoV-2, the virus that the researchers focused on in their study. Neutralizing antibodies, which can be generated by vaccination or a previous infection, offer protection against future infections.
“This type of assay could be used to check whether patients are really protected by vaccines or not,” says Laura Kiessling, the Novartis Professor of Chemistry at MIT and the senior author of the paper. “If someone is at high risk, it would be really good to be able to rapidly determine if they have neutralizing antibodies.”
This technique, which uses general equipment already found in many biochemistry labs, could also help researchers to determine how well current vaccines might protect against emerging variants of SARS-CoV-2, Kiessling says.
Former MIT postdoc Michael Wuo and MIT research scientist Amanda Dugan are the lead authors of the paper, which appears today in the open-access journal ACS Central Science.
Neutralizing or not?
Most vaccines for SARS-CoV-2 target the spike protein of the virus, which the virus uses to enter host cells through the ACE2 receptor. Like most proteins found on viral envelopes, the spike protein is heavily coated in sugar chains that hang from the protein.
Kiessling, whose lab studies how proteins interact with carbohydrates found on cell surfaces, wondered if it might be possible to create a “fingerprint” of different antibodies, based on how they interact with the sugar molecules found on a viral protein such as the SARS-CoV-2 spike protein.
“To tell whether an antibody is neutralizing or not, you usually have to do a relatively difficult set of assays,” Kiessling says. “You have to test whether or not the antibody blocks the virus from infecting cells. We thought if we could develop this fingerprint, then we could identify neutralizing antibodies much more rapidly.”
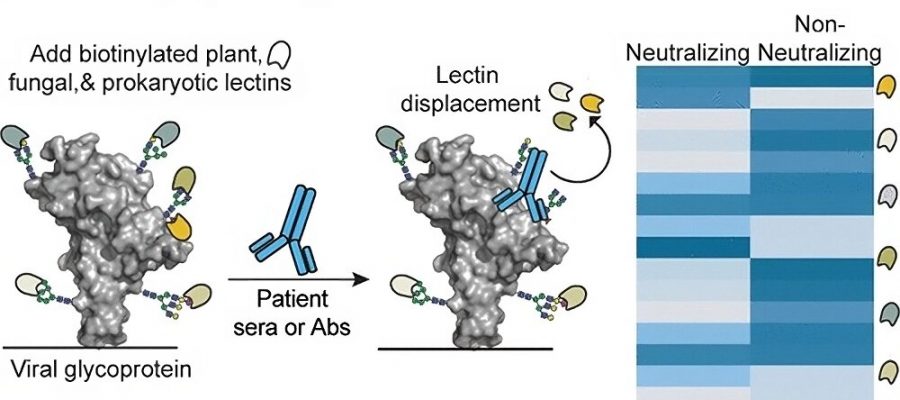
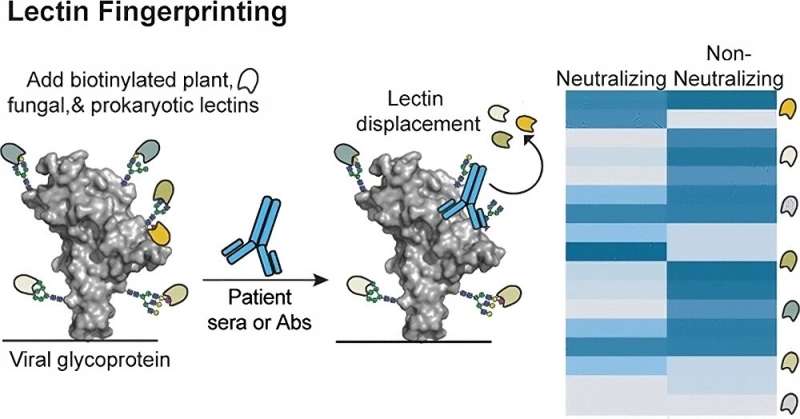
To do that, the researchers created a panel of commercially available lectins (proteins that bind to carbohydrates), taken from a variety of organisms, mostly plants and bacteria. Lectins, which are normally involved in functions such as cell-cell interactions and immune responses, bind to the sugar molecule at the very end of a sugar chain as it dangles from a protein.
When the researchers expose the SARS-CoV-2 spike protein to these lectins, each lectin attaches to a particular subset of sugar molecules found on the protein. Then, the researchers add serum containing antibodies against SARS-CoV-2. If the antibodies have a high affinity for the spike protein, they jostle the lectins already there out of the way.
Each antibody displaces a different set of lectins, depending on its binding specificity, and this displacement can be measured using a laboratory test known as enzyme-linked lectin assay (ELLA). By analyzing whether each antibody displaced 28 different lectins bound to the spike protein, the researchers were able to identify patterns of lectin displacement, creating a distinctive “fingerprint” for each antibody.
The researchers first identified fingerprints for antibodies that were already known to be either neutralizing or non-neutralizing. Then, they tested patient blood samples and were able to determine whether antibodies from those samples were neutralizing or not, by comparing them to the fingerprints produced by the known neutralizing antibodies.
“By looking at the different patterns, we could see that neutralizing antibodies fell into a different category as the non-neutralizing antibodies,” Kiessling says.
Antibody profiles
Using this analysis, the researchers were also able to categorize antibodies based on whether they came from people who received the Moderna COVID-19 vaccine or the Pfizer COVID-19 vaccine, each of which targets slightly different viral RNA sequences.
The researchers have filed for a patent on the technology, which they hope could be developed to perform rapid tests in a doctor’s office to determine the antibody profile of individual patients.
This technique could potentially be adapted to identify neutralizing antibodies against new variants of SARS-CoV-2, or other disease-causing viruses, Kiessling says. Now that the researchers have a panel of lectins that can be used for the test, they would simply need to re-run the analysis with antibodies that are known to be neutralizing and non-neutralizing, so they can determine the correct fingerprint for those antibodies.
“We could use the same panel of lectins for all SARS-CoV-2 variants of concern,” Kiessling says. “It can be useful for any new viruses that emerge, as long as they have a viral envelope.”
More information:
Michael G. Wuo et al, Lectin Fingerprinting Distinguishes Antibody Neutralization in SARS-CoV-2, ACS Central Science (2023). DOI: 10.1021/acscentsci.2c01471. pubs.acs.org/doi/10.1021/acscentsci.2c01471
Journal information:
ACS Central Science
Source: Read Full Article