Researchers in the United States have demonstrated the effectiveness of three antibody agents at neutralizing recently emerged variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent responsible for the coronavirus disease 2019 (COVID-19) pandemic.
The team showed that one full-length antibody and two antibody domains each effectively neutralized pseudotyped virus containing key mutations found within the B.1.1.7 variant that emerged in the UK.
However, only one of the antibody domains was effective at neutralizing pseudotyped virus containing key mutations found within the South African B.1.351 lineage.
“The results inform the further clinical development of these antibodies against emerging SARS-CoV-2 variants, either by making antibody cocktails or by constructing bispecific antibodies,” writes Zehua Sun and colleagues from the University of Pittsburgh Medical School in Pennsylvania.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
.jpg)
Emerging variants threaten current therapeutic approaches
The transmission of recently emerged SARS-CoV-2 variants remains uncontrolled in many countries. Studies have shown that monoclonal antibodies (mAbs) and vaccine-induced antibodies are less effective at neutralizing these variants, including the UK B.1.1.7 lineage, the South African B.1.351 and the Brazilian P.1 lineage.
The initial stage of the SARS-CoV-2 infection process is mediated by the viral spike protein. The receptor-binding domain (RBD) of the spike attaches to the human host cell receptor angiotensin-converting enzyme 2 (ACE2) to enable viral fusion and cell entry.
Researchers have been developing mAbs and antibody-eliciting vaccines that neutralize infection through antibody binding of the spike RBD to prevent its interaction with ACE2.
However, a number of mutations have been characterized in the new variants that stop these neutralizing antibodies from binding to the spike RBD.
How many mutations in spike and the RBD have the different variants evolved?
The UK variant contains eight spike mutations, one of which (N501Y) is located in the RBD, while the South African variant contains nine spike mutations, with three (K417N, E484K and N501Y) located in the RBD. The Brazilian variant also contains this triple mutation (K417T, E484K, and N501Y) in the RBD.
“These mutations contribute to an increased affinity of the RBD for human ACE2, improving viral entry into cells,” says Sun and colleagues. “The rapid emergence of new variants is of great concern for their increased transmissibility and evasion of neutralizing antibody therapeutics and the humoral response generated by currently approved vaccines.”
Furthermore, although some of the mutations in these variants only partially reduce neutralization by vaccinated sera or currently approved mAbs, the constantly evolving variants could become dominant and more efficient at evading therapeutics and vaccines, adds the team.
The researchers previously identified one full-length immunoglobulin 1 antibody (ab1) and two antibody domains (ab6 and ab8) that exhibited potent neutralizing activity against the RBD of the wild-type SARS-CoV-2 spike protein.
“Ab1, ab6, and ab8 are all antibody or antibody domains that can compete with human ACE2 for binding to the wild-type SARS-CoV-2 RBD,” they write.
What did the current study involve?
The researchers evaluated the effectiveness of these previously identified antibody agents at binding and neutralizing the SARS-CoV-2 variants. They performed an ELISA assay against a panel of RBDs containing key mutations and performed pseudotyped virus neutralization assays.
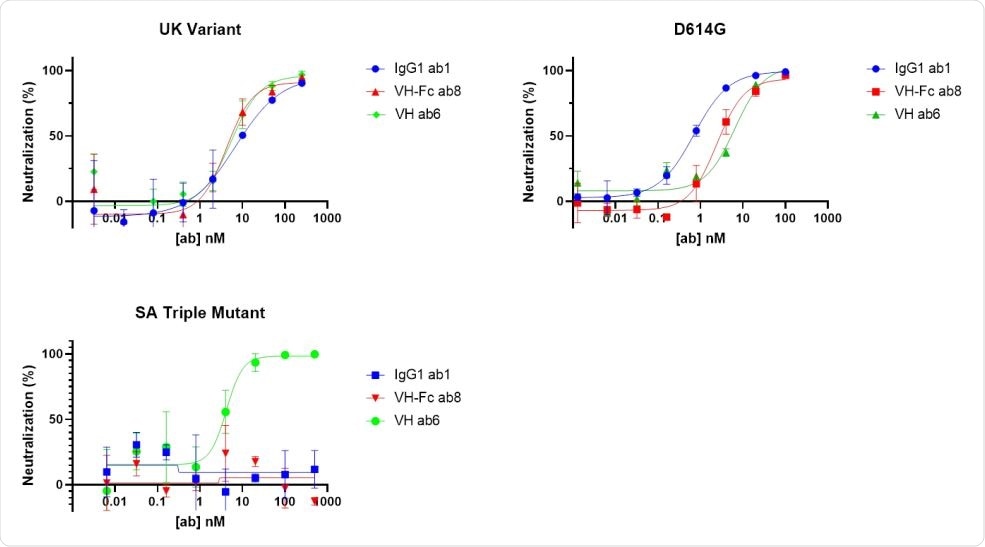
The team found that the antibody domain ab6 bound to 32 of 35 RBD mutants tested in the ELISA assay, including the triple mutation (K417N, E484K and N501Y) found in the South African and Brazilian variants.
However, this triple mutation completely blocked binding by the full-length ab1 antibody and the ab8 antibody domain.
“These observations indicate that ab1 and ab8 may completely lose neutralization of the B.1.351 variant,” says the team.
Ab6 also neutralized pseudotyped viruses with spike proteins containing the B.1.1.7 mutations and spikes containing the B.1.351 mutations.
By contrast, ab1 and ab8 only neutralized the B.1.1.7 variant and not the triple-mutant variant.
What do the authors conclude?
“Antibody domain ab6 exhibited potency in neutralizing all the pseudotyped variants bearing these mutations,” says the team.
The researchers say that vaccination is still an efficient approach to the long-term control of SARS-CoV-2 variant transmission.
However, “our study provides highly potent antibody candidates that neutralized emerging mutant variants that can be used as emergency therapeutics where current vaccines are not present or less effective in prevention,” concludes Sun and colleagues.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Sun Z, et al. Neutralization of European, South African, and United States SARS-CoV-2 mutants by a human antibody and antibody domains. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.03.22.436481, https://www.biorxiv.org/content/10.1101/2021.03.22.436481v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Assay, Cell, Coronavirus, Coronavirus Disease COVID-19, Enzyme, Immunoglobulin, Medical School, Mutation, Pandemic, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Therapeutics, Vaccine, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article
