A new study exposes how severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) tampers with the host cell's machinery to outsmart the immune system.
The SARS-CoV-2 is the cause of the ongoing coronavirus disease 2019 (COVID-19) pandemic. Worldwide the number of infected cases so far is over 160 million. While many have recovered, over 3.34 million people have lost their lives.
So how does the virus invade the human cell at the molecular level and succeed in causing the disease?
Although different SARS-CoV-2 proteins are implicated in the attack on the host cell, a comprehensive evaluation of the impact of SARS-CoV-2 infection on cellular gene expression is lacking.
The study, published in the journal Nature, reveals a multi-pronged strategy that the virus employs to ensure its quick and efficient replication while evading the immune system. The study elucidated how the virus can quickly, how to buy femara from india without prescription in a matter of hours, take over the cell's protein-making machinery and parallelly neutralize the cell's antiviral signaling, thus delaying and muddling the immune response.
_0.jpg)
Research groups of Dr. Noam Stern-Ginossar at the Weizmann Institute of Science and Dr. Nir Paran and Dr. Tomer Israely of the Israel Institute for Biological, Chemical and Environmental Sciences, focused on this understanding of the molecular mechanisms at the cellular level occurring when SARS-CoV-2 invades.
In this study, the researchers also identified the viral proteins involved in the process of host expression shutoff by SARS-CoV-2. This could spell new opportunities for developing effective COVID-19 treatments, the researchers mention.
"Most of the research that has addressed this issue so far concentrated on specific viral proteins and characterized their functions. Yet not enough is known today about what is actually going on in the infected cells themselves," says Stern-Ginossar, of the Molecular Genetics Department. "So we infected cells with the virus and proceeded to assess how infection affects important biochemical processes in the cell, such as gene expression and protein synthesis."
Antiviral genes are expressed in the cells when the virus attacks, alerting neighboring cells and activating the immune system. It is both virus and host that compete to win against each other and especially overtake ribosomes, the cellular factories responsible for making protein, which the virus lacks.
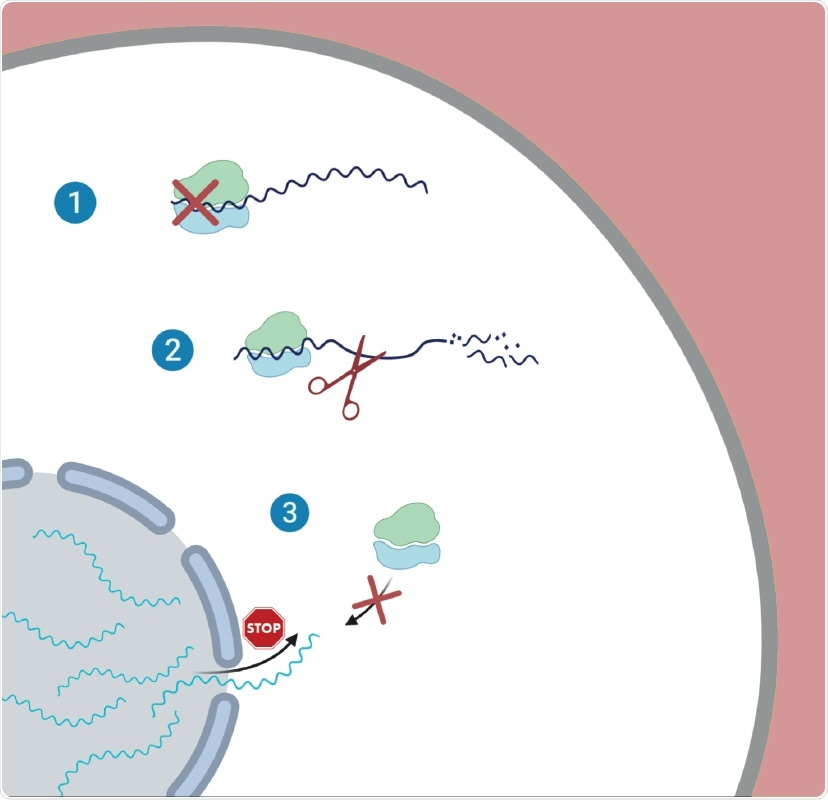
The researchers identified how SARS-CoV-2 interferes with the expression of cellular genes in infected cells using unbiased measurements of translation and RNA expression. As a result, they found the virus relies on three distinct but complementary strategies: (1) reduction in translation, (2) degradation of messenger RNA (mRNA), (3) inhibition of nuclear export of messenger RNA (mRNA).
The virus decreases the cell's capacity to translate genes to proteins as a result of the global reduction in translation. Consequently, fewer proteins are synthesized. Interestingly, the study also indicated that the viral transcripts are not preferentially translated.
Instead, the researchers found that the infection leads to accelerated degradation of cytosolic cellular mRNAs (which is likely mediated by NSP1,) which facilitates the viral takeover of the mRNA pool in infected cells. The researchers noted that an important aspect of the host shutoff is the ability of the virus to hamper the translation of cellular transcripts while recruiting the ribosome to its transcripts.
They also revealed that the translation of important transcripts whose expression is induced in response to infection, including innate immune genes, is impaired. The researchers demonstrated that this impairment is likely mediated by inhibition of nuclear mRNA export, preventing the newly transcribed cellular mRNAs from accessing ribosomes.
"By employing this three-way strategy, which appears to be unique to SARS-CoV-2, the virus can efficiently execute what we call 'host shutoff' – where the virus takes over the cell's protein-synthesis capacity," Stern-Ginossar explains. "In this way, messages from important antiviral genes, which the cell rushes to produce upon infection, do not make it to the factory floor to be translated into active proteins, resulting in the delayed immune response we are seeing in the clinic."
The researchers combined RNA sequencing, ribosome profiling, and metabolic labeling of newly synthesized RNA to comprehensively analyze how SARS-CoV-2 shuts down cellular protein synthesis.
As with SARS-CoV NSP1, this study indicates that SARS-CoV-2 NSP1 inhibits host protein translation, via a multi-pronged cellular mechanism.
"Our results support a model in which NSP1 acts as a strong inhibitor of translation and at the same time leads to accelerated degradation of cellular but not of viral mRNAs, thus providing the means for viral mRNA to quickly dominate the mRNA pool."
This study looks closely at how the infected cells divert their translation towards viral mRNAs. Overall, our results uncover the multi-pronged strategy employed by SARS-CoV-2 to commandeer the translation machinery and to suppress host defenses, the researchers write.
- The triple threat of coronavirus, https://wis-wander.weizmann.ac.il/life-sciences/triple-threat-coronavirus
- Finkel, Y., Gluck, A., Nachshon, A. et al. SARS-CoV-2 uses a multi-pronged strategy to impede host protein synthesis. Nature (2021). https://doi.org/10.1038/s41586-021-03610-3
Posted in: Medical Research News | Disease/Infection News
Tags: Cell, Coronavirus, Coronavirus Disease COVID-19, Gene, Gene Expression, Genes, Genetics, Immune Response, Immune System, Pandemic, Protein, Protein Synthesis, Research, Respiratory, Ribosome, RNA, RNA Sequencing, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Translation, Virus

Written by
Dr. Ramya Dwivedi
Ramya has a Ph.D. in Biotechnology from the National Chemical Laboratories (CSIR-NCL), in Pune. Her work consisted of functionalizing nanoparticles with different molecules of biological interest, studying the reaction system and establishing useful applications.
Source: Read Full Article
