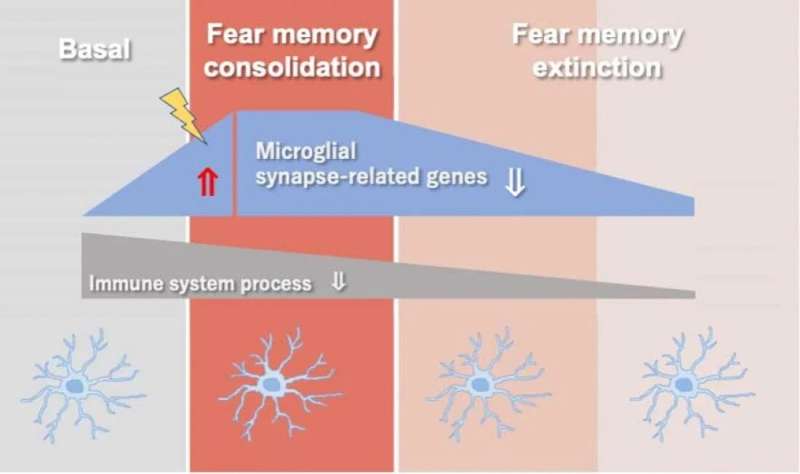
Microglia are the first line of defense in the central nervous system, constantly scanning for pathogens and abnormalities and releasing small proteins called cytokines to wade off infections. Previous research has shown that, in mice conditioned to fear a particular environment (contextual fear conditioning), microglia play a pivotal role in transferring traumatic memories from short to long-term memory (fear memory consolidation), and the memories’ subsequent extinction.
Now, Tohoku University scientists have demonstrated that microglial genes associated with the synapse—structures that allow neurons to pass signals to one another—undergo changes in response to the consolidation and extinction of contextual fear conditioning. This suggests that microglia and neurons crosstalk via “non-immune” functions and clarifies the mechanisms linking microglia and neuronal activity related to fear conditioning.
Details of their research were published in the journal Brain Research Bulletin on August 18, 2022.
Dr. Zhiqian Yu and Professor Hiroaki Tomita from Tohoku University’s Graduate School of Medicine and their team has previously revealed that when mice were subjected to chronic and acute stress, their microglial released a type of cytokine known as TNF-a, which is used by the immune system for cell signaling. TNF-a increased during fear memory consolidation, but returned to base levels after extinction. Hippocampal TNF-a, furthermore, blocks the retrieval and reconsolidating of contextual fear and spatial memories.
Building on the previous study, the team adopted microarray techniques in microglia from mice exposed to contextual fear conditioning. They showed that synapse-related genes in microglia are changed by contextual fear conditioning. However, they also discovered that consolidating the fear memory induced immune dysfunction in microglia and did not recover even during the process of extinction.
Within the microglia’s plethora of synaptic function-related genes, Gamma-aminobutyric acid (GABA) and GABAR receptors (GABAR) are the earliest neurotransmitter systems to emerge during development. The GABARB3 encodes the β3 subunit of GABAA receptors in neurological disorders such as epilepsy and autism.
“Using real-time PCR and immune stain technologies, we found that GABRB3 was expressed in microglial cytoplasm and the long branching processes of the hippocampus,” said Yu. “The mRNA and protein levels of GABRB3 changed significantly after fear memory consolidation but recovered after extinction.”
Additionally, the researchers investigated a family of proteins called Synapsin, which regulate neurotransmitters’ release at the synapse. The transcription of microglial Synapsin was expressed in MG-6 cell line and primary microglia that increased under fear memory consolidation but recovered after fear memory extinction.
Source: Read Full Article
