Proteins, long polymers comprised of smaller constituents known as amino acids, play a crucial role in the functioning of the human body. Over the course of a human’s life, these “strings” of proteins fold into unique 3D structures or conformations, and this folding process affects how different channels and receptors in the brain interact with other proteins.
As humans get older, genetic mutations and environmental factors can cause the misfolding of proteins. Neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, are now known to be caused by the incorrect “folding” of proteins.
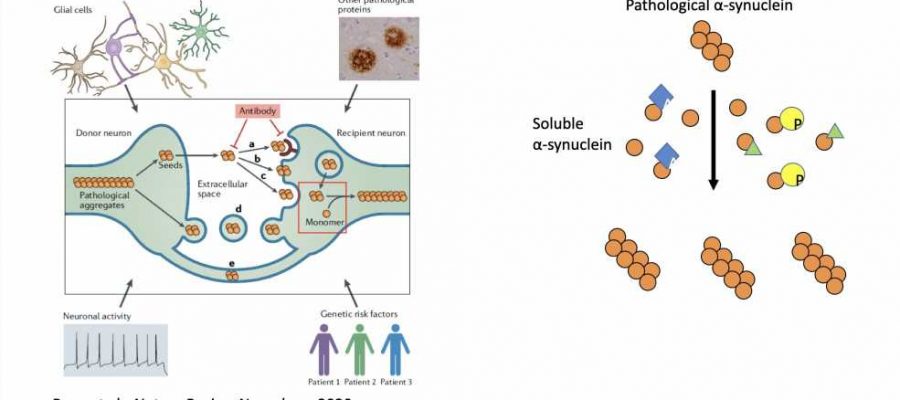
A team of researchers at University of California, Los Angeles (UCLA), University of Pennsylvania, and other institutes in the U.S. and China recently uncovered a mechanism that regulates the transmission of a pathological protein, misfolded alpha-synuclein, which has been found to be associated with the progression of Parkinson’s disease. This mechanism, outlined in a paper published in Nature Neuroscience, consist in a series of alterations that a cell can make to these proteins, which affects their ability to spread in the brain.
“The whole idea for the paper developed when I was writing a review for the field, where I summarized all the known mechanisms for the transmission of pathological proteins,” Chao Peng, one of the researchers who carried out the study, told Medical Xpress.
“As I was writing it, I noticed is that current research of pathological protein transmission has primarily focused on the pathological protein, or the ‘seed.’ However, successful spreading of pathological protein requires not only the pathological protein (the seed), but also requires the corresponding normal soluble protein (the substrate).”
Most past neuroscience studies focusing on proteins and neurodegenerative diseases did not explore the mechanisms through which soluble proteins may modulate amplification of pathological proteins. The key objective of the recent work by Peng and his colleagues was to investigate these effects, specifically focusing on the protein alpha-synuclein (a-syn), which has been linked to the progression of Parkinson’s disease when misfolded.
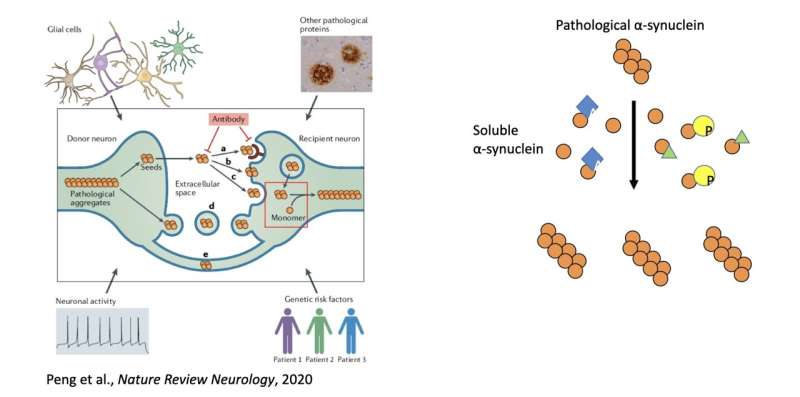
“Many post-translational modification (PTMs) has been discovered on a-syn, many of which have been discovered on soluble a-syn as well,” Peng said. “Therefore, we decided to test whether soluble a-syn PTMs would modulate the amplification of pathological a-syn. We thus developed a cell-based assay that we can use to mimic this amplification process in cells.”
In their experiments, the researchers used pathological versions of the protein a-syn that matched those observed in patients with different neurodegenerative diseases. They then analyzed the amplification of these pathological a-syn proteins in different diseases.
Overall, the findings gathered by Peng and his colleagues pinpoint a novel mechanism through which soluble, altered a-syn proteins affect the amplification of pathological a-syn, the protein associated with the progression with Parkinson’s and other neurodegenerative diseases. In the future, they could thus inspire additional studies exploring this newly identified mechanism, while also potentially informing the development of new targeted therapeutic interventions.
“Our study is the first to analyze how the soluble protein would modulate the amplification of pathological protein, which represents a completely novel mechanism to modulate pathological protein spreading in diseased brains,” Peng added. “Even though this study focuses on a-syn, we believe the same mechanism is applicable to other neurodegenerative diseases related pathological proteins as well. We now plan to study how soluble proteins modulate the amplification of pathological proteins for other neurodegenerative disease-related pathological proteins.”
More information:
Shujing Zhang et al, Post-translational modifications of soluble α-synuclein regulate the amplification of pathological α-synuclein, Nature Neuroscience (2023). DOI: 10.1038/s41593-022-01239-7
Chao Peng et al, Protein transmission in neurodegenerative disease, Nature Reviews Neurology (2020). DOI: 10.1038/s41582-020-0333-7
Journal information:
Nature Reviews Neurology
,
Nature Neuroscience
Source: Read Full Article