Vanderbilt researchers have demonstrated that a cluster of microRNAs—small pieces of RNA that regulate gene expression—work in a type of immune cells called macrophages to help protect against metabolic defects in obesity.
The findings, published in the journal Cell Reports, provide a glimpse into the function of macrophages in fat tissue and could open new therapeutic avenues to prevent diseases associated with obesity, said Heather Pua, MD, Ph.D., assistant professor of Pathology, Microbiology and Immunology and senior author of the new study.
“We have to understand the details of cellular and molecular pathogenesis in obesity to ultimately design therapeutic interventions—in this case that could be targeting the microRNAs or the genes and signaling pathways they impact,” Pua said.
Pua and her group were interested in macrophages because previous studies have found that they are the most abundant type of immune cell in adipose tissue.
“There’s an emerging literature over the past 20 years that immune cells play critical roles in adipose in regulating our metabolic health and responses to obesity,” Pua said. “Our goal has been to try to understand how immune cells either protect from or contribute to disease.”
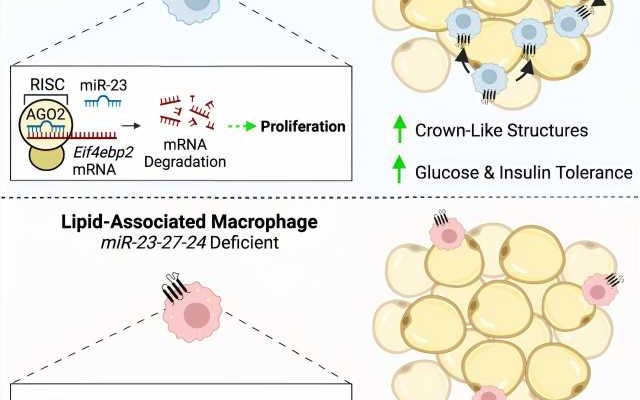
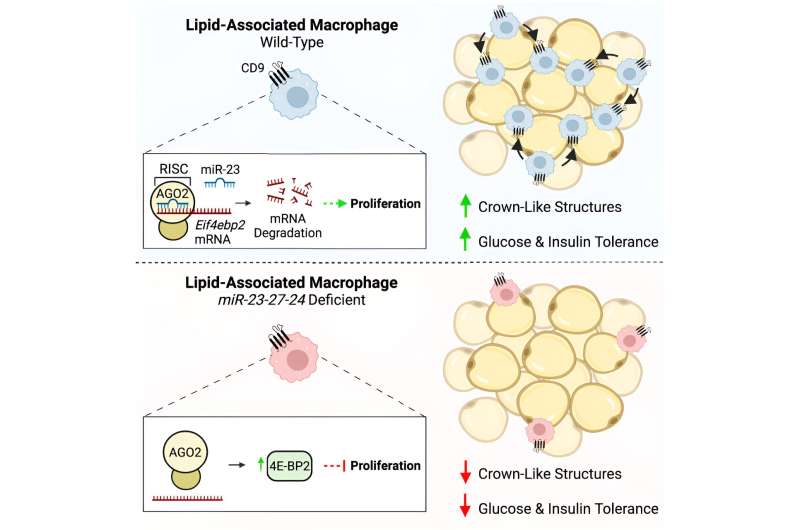
In studies led by Neil Sprenkle, Ph.D., who was a graduate student in the lab, the researchers deleted the expression of a cluster of microRNAs in mouse myeloid cells, which include macrophages. When these mice ate a high-fat diet, they gained less weight than mice with the microRNAs, but they had worse metabolic outcomes, including worse glucose and insulin intolerance.
The researchers found that mice missing the microRNAs had fewer macrophages in their adipose tissue.
“That finding was a bit counterintuitive because macrophages are often thought to be pro-inflammatory and contribute to insulin resistance,” Pua said. “We’re understanding now that it’s complex and that there are some macrophages that serve protective roles, especially late in obesity.
“We think that this cluster of microRNAs is important for promoting the proliferation of a protective subset of macrophages that helps the adipose tissue adapt to the stress of obesity. When you take these microRNAs away, these cells don’t proliferate, and you lose this protection.”
The researchers used methods including RNA sequencing, functional screens and biochemical assays to identify the gene networks regulated by the microRNAs. They zeroed in on one gene in a pathway with known roles in nutrient handling, growth and proliferation.
“We’ve identified one gene, but I guarantee it’s not the only gene involved because microRNAs always act through networks of genes,” Pua said.
The researchers will continue to explore this microRNA-regulated network and how the signaling pathways it controls are important for cell proliferation and function.
“Our goal is to go from the organismal level to a change in a cell type to the molecular underpinnings of that change,” Pua said.
More information:
Neil T. Sprenkle et al, The miR-23-27-24 clusters drive lipid-associated macrophage proliferation in obese adipose tissue, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112928. www.cell.com/cell-reports/full … 2211-1247(23)00939-7
Journal information:
Cell Reports
Source: Read Full Article