A research team led by Professor Stephanie Ma Kwai-yee from School of Biomedical Sciences, LKS Faculty of Medicine, the University of Hong Kong (HKUMed) has uncovered a novel signaling pathway by which RNA editing governs lipid metabolism to promote resistance to chemotherapy and cancer stemness in gastric cancer. The findings have been published in Nature Communications.
Gastric cancer (GC) remains one of the leading causes of cancer-related deaths both globally and in Hong Kong. To combat GC, 5-fluorouracil (5-FU) and platinum-based combination chemotherapy is often administered in addition to surgical resection, in the hope of increasing the effectiveness of surgery or to minimize the chance of cancer recurrence. However, the emergence of acquired chemoresistance eventually curtails the long-term clinical benefits.
The myriad mechanisms driving chemoresistance, compounded with GC constituting various subtypes obscure the identification of targets to override chemoresistance. Therefore, understanding subtype-specific vulnerability to override resistance to chemotherapy is fundamental to designing improved treatment options for this deadly disease.
Research method and findings
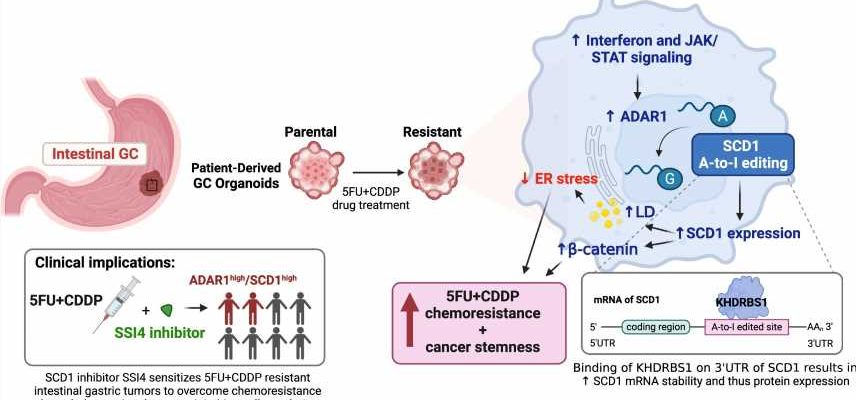
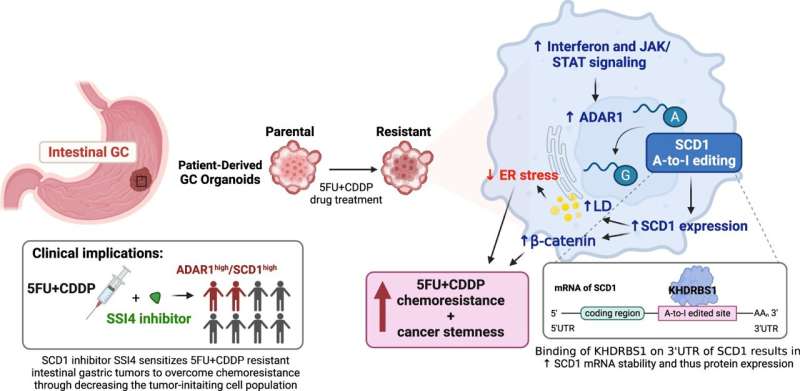
The research team discovered an unreported mechanism driving chemoresistance by which dysregulated editing at the RNA level instigates aberration in lipid metabolism to endow chemoresistance and cancer stemness.
By integrating clinical intestinal gastric cancer patient-derived organoid lines trained to mimic 5-FU+ cisplatin resistance, multi-omic profiling and validation in pre-clinical GC mouse models, the research team found that the chemotherapy-resistant organoids displayed greater interferon and JAK/STAT signaling, which upregulated ADAR1 expression. ADAR1, an enzyme that dictates the editing of RNA, fosters a dysregulated lipid network via the editing of multiple enzymes involved in lipid metabolism, including a key enzyme, SCD1.
The editing of SCD1 RNA enhanced the stability of mRNA and thereby increased SCD1 protein expression. Consequently, SCD1 facilitated lipid droplet formations and β-catenin abundance to confer resistance to chemotherapy as well as a more cancer stemness state. To apply the findings for clinical use, the team further demonstrates that supplementation of an SCD1 inhibitor (SSI4) to a chemotherapy regimen could reverse chemoresistance in gastric cancer and reduce the tumor-initiating subset.
“Our findings identified dysregulated editing at the RNA level of lipid metabolic genes as a novel molecular mechanism underlying resistance to chemotherapy in gastric cancer. By targeting edited SCD1, we can reverse chemoresistance and cancer stemness. This research has laid the foundation for the future development of new treatments for this deadly disease,” said Professor Stephanie Ma Kwai-yee of the School of Biomedical Sciences, HKUMed, who initiated the study.
“Further, ADAR1 expression and SCD1 may also be good biomarkers for predicting response to chemotherapy in gastric cancer patients. This spares the patients from going through unnecessary chemotherapy and allows them to carry on more effective treatment,” said Kwai-yee.
More information:
Tin-Lok Wong et al, ADAR1-mediated RNA editing of SCD1 drives drug resistance and self-renewal in gastric cancer, Nature Communications (2023). DOI: 10.1038/s41467-023-38581-8
Journal information:
Nature Communications
Source: Read Full Article