Coronavirus disease 2019 (COVID-19) disproportionately affects patients 65 years and older. Age-related impaired host immunity has been implicated for severe outcomes of COVID-19. Researchers have performed a study on large data set to understand better the molecular basis of vulnerability in aged COVID-19 patients. They observed certain age-linked immunoinflammatory factors associated with severe COVID-19 and could potentially be targeted therapeutically to reduce morbidity and mortality in aged patients.
 Study: Aging-related cell type-specific pathophysiologic immune responses that exacerbate disease severity in aged COVID-19 patients. Image Credit: Ann Kosolapova/ Shutterstock
Study: Aging-related cell type-specific pathophysiologic immune responses that exacerbate disease severity in aged COVID-19 patients. Image Credit: Ann Kosolapova/ Shutterstock
Reports show that when compared to younger individuals (18-55 years), aged individuals with COVID-19 show disrupted adaptive immunity to SARS-CoV-2, in the form of reduced coordination of CD4-CD8 T cell response. Aged individuals have also been reported to produce a weaker type I interferon (IFN) response to Influenza virus infection, indicating disrupted innate immune antiviral defense. In addition, aberrant immunosenescence leading to hyper-inflammation in response to pathogen-associated molecular patterns (PAMPs) have also been implicated in age-mediated COVID-19 severity.
A preprint version of the study is available on the medRxiv* server while the article undergoes peer review.
Serious consequences in aged COVID-19 patients
The scientists assessed epidemiological data from March to December 2020 from U.S. Centers for Disease Control (CDC) to investigate the prevalence of COVID-19 disease among different age groups. The nine-month data set analysis revealed that 80.5% of deaths occurred in aged patients, 19.5% in 18-64 years old (19.5%), and only 0.05% in the 0-17 years old age group.
Subsequently, the authors used odds ratio (OR) to measure the association between aging and COVID-19 outcomes. From the U.S. CDC datasets adjusted for the confounding factors of sex and race (OR Model-1), aged individuals showed a significantly increased likelihood of COVID-19-related hospitalization (OR = 9.07, 95% confidence interval [CI] 9.99 – 9.15), ICU admission (OR = 9.24, 95% CI 9.01 – 9.48), and death (51.15, 95% CI 49.86 – 52.47).
OR Model-2 was tested in the COVID-19 registry data from Cleveland Clinic in Ohio and Florida, adjusted for sex, race, smoking, hypertension, diabetes, coronary artery disease, asthma, and emphysema to account for disease comorbidities and chronic obstructive pulmonary disease. The aged individuals had significantly greater likelihood of COVID-19-related hospitalization (OR = 3.10, 95% CI 2.55 – 3.77), ICU admission (OR = 2.39, 95% CI 1.78 – 3.22), and death (OR = 40.35, 95% CI 19.80 – 82.24).
Elevated inflammatory markers and pro-inflammatory cytokines in aged COVID-19 patients
Analysis on the Cleveland Clinic COVID-19 registry dataset detected elevated levels of D-dimer, C reactive peptide (CRP) and Neutrophil-lymphocyte ratio (NLR), inflammatory markers associated with severity and death in COVID-19, in aged hospitalized COVID-19 patients. Pro-inflammatory cytokine IL-6 was elevated in both young and aged patients. Being a critical mediator of cytokine storm in COVID-19, IL-6 is a potential therapeutic target. However, a recent Phase III clinical trial failed to show reduced mortality in severe COVID-19 patients treated with the anti-IL-6R monoclonal antibody tocilizumab.
Furthermore, the hospitalized aged patients exhibited upregulation of pro-inflammatory cytokines IL-8 and IL-27, the hospitalized younger patients exhibited the upregulation of IL-10 expression. These observations signify distinct inflammatory cytokine profiles between aged and younger COVID-19 patients, potentially mediating age-related hospitalization and ICU admission.
Declined naïve CD8 T cell numbers and IFN deficiencies in aged severe COVID-19 patients
Deep immune profiling on T cell data revealed significantly fewer naive CD8 T cells in hospitalized aged patients. Naive CD8 T cell-mediated homeostasis is an important antiviral defense component, and a reduced abundance of naïve CD8 T cells could dysregulate adaptive immunity. Further, single-cell RNA seq (scRNA-seq) data on CD8 T cells detected the upregulation of the apoptosis gene, cathepsin D27 (CTSD), in aged severe COVID-19 patients.
At the same time, interferon-stimulated genes IFITM3 and TRIM22, enriched in type I & II IFN signaling pathways, were downregulated. In addition, the transcription factor STAT1, an important downstream factor in type I & II IFN signaling pathways, was downregulated in CD8 naive T cells in aged COVID-19 patients. The team suggests that IFN deficiencies in CD8 naive T cells may contribute to the increased severity of COVID-19 disease in aged patients.
Age-related correlation between Interferon deficiencies and SARS-CoV-2 viral load
Expression levels of IFNα genes (IFNA1, IFNA5, IFNA7 and IFNA8) and the IFN-stimulated antiviral genes IFIT1 and OAS1 (2'-5'-Oligoadenylate Synthetase) were significantly down-regulated in aged patients with high viral load, as analyzed on bulk RNA-seq data from nasopharyngeal samples. IFN is a potent inhibitor of pro-inflammatory cytokine IL-8 in viral infections, and so these findings corroborate with elevated plasma levels of IL-8 in aged COVID-19 patients.
Elevated expression of SARS-CoV-2 entry factors in aged patients
The team observed a reduced abundance of angiotensin-converting enzyme-2 (ACE2) on secretory and ciliated cells in aged COVID-19 patients. However, the more recently identified SARS-CoV-2 docking receptor basigin (BSG or CD147) was expressed in 95% of secretory cells in aged patients with critical COVID-19, particularly in regulatory T cells (Treg) and CTLs. Treg and CTLs also showed increased levels of S-protein priming protease FURIN. The team suggests that elevated expression of two SARS-CoV-2 factors, BSG and FURIN, in Treg and CLT cells may contribute to the increased susceptibility of aged patients to COVID-19.
Increased immune-epithelial cell interactions in aged COVID-19 patients
Applying CellphoneDB, the team demonstrated elevated expression of TGF-β genes (TGFB1, TGFB2, and TGFB3) and their receptors (TGFBR2 and TGFBR3) in both secretory Treg and non-resident macrophages (nrMa). TGF-β regulates the chronic immune response to SARS-CoV-2 in severe COVID-19 patients. Thus Cheng and the team suggest that higher levels of TGF- β and their receptors may explain the long duration of hospitalization in aged COVID-19 patients.
In addition, secretory/ciliated – CTL cell interaction in aged patients showed much weaker IFNG – IFNGR interaction than young patients with COVID-19. The team suggests that immune-epithelial cell interactions are associated with critical COVID-19 in aged patients. In particular, reduced expression of IFNR signaling is associated with greater severity of COVID-19 in aged individuals.
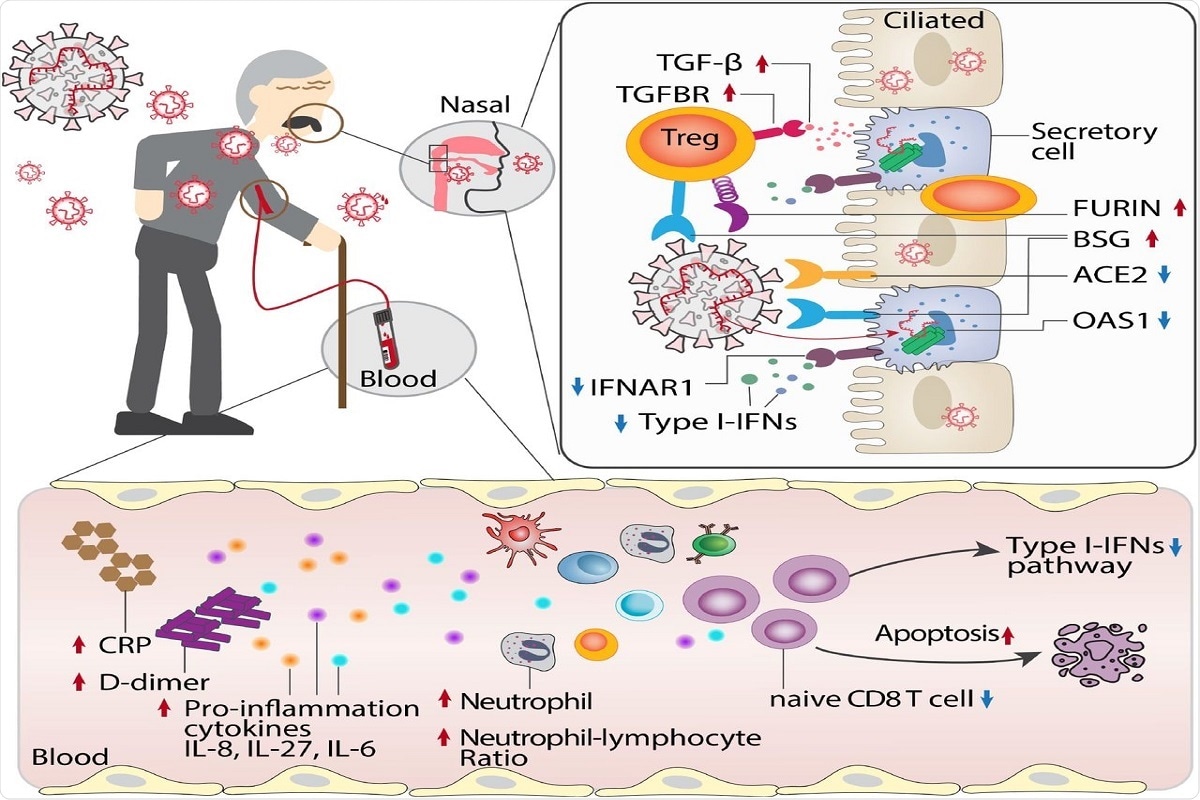 Figure 6. Proposed mechanistic models for age-biased COVID-19 severity in aged individuals. Several age-related pathophysiologic immune responses are associated with disease susceptibility and severity in COVID-19: a) decreased lymphocyte count and elevated inflammatory markers (C-reactive protein [CRP], D-dimer, and neutrophil-lymphocyte ratio); b) elevated pro-inflammation cytokines IL-8, IL-27 and IL-6 in aged COVID19 patients; c) reduced abundance of naïve CD8 T cells with decreased expression of antiviral defense genes (i.e., IFITM3 and TRIM22) in aged individuals with severe COVID-19; d) type I interferon deficiency is associated with SARS-CoV-2 viral load in aged individuals; e) elevated expression of SARS-CoV-2 entry factors (BSG and FURIN) and reduced expression of antiviral defense genes (IFNAR1, OAS1, IFIT1) in the secretory cells of critical COVID-19 in aged individuals; f) strong TGF-beta mediated immune-epithelial cell interactions (i.e., secretory – T regulatory cells) in aged individuals with critical COVID-19.
Figure 6. Proposed mechanistic models for age-biased COVID-19 severity in aged individuals. Several age-related pathophysiologic immune responses are associated with disease susceptibility and severity in COVID-19: a) decreased lymphocyte count and elevated inflammatory markers (C-reactive protein [CRP], D-dimer, and neutrophil-lymphocyte ratio); b) elevated pro-inflammation cytokines IL-8, IL-27 and IL-6 in aged COVID19 patients; c) reduced abundance of naïve CD8 T cells with decreased expression of antiviral defense genes (i.e., IFITM3 and TRIM22) in aged individuals with severe COVID-19; d) type I interferon deficiency is associated with SARS-CoV-2 viral load in aged individuals; e) elevated expression of SARS-CoV-2 entry factors (BSG and FURIN) and reduced expression of antiviral defense genes (IFNAR1, OAS1, IFIT1) in the secretory cells of critical COVID-19 in aged individuals; f) strong TGF-beta mediated immune-epithelial cell interactions (i.e., secretory – T regulatory cells) in aged individuals with critical COVID-19.
Study limitations
Although the team inspected -omics data from multiple tissues, including PBMCs, plasma, and nasal tissues, they warrant additional analysis of other COVID-19 and aging pertinent tissues, such as lung and brain.
The current study findings are based on data generated from acute COVID-19 patients only. The team advises the need to study data on chronic COVID-19 patients.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Hou, Y. et al. (2021) "Aging-related cell type-specific pathophysiologic immune responses that exacerbate disease severity in aged COVID-19 patients". medRxiv. doi: 10.1101/2021.09.13.21263504.
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Aging, Angiotensin, Antibody, Apoptosis, Asthma, Brain, CD4, Cell, Chronic, Chronic Obstructive Pulmonary Disease, Clinical Trial, Coronary Artery Disease, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Cytokines, D-dimer, Diabetes, Emphysema, Enzyme, Gene, Genes, Immune Response, immunity, Inflammation, Influenza, Interferon, Lymphocyte, Monoclonal Antibody, Mortality, Pathogen, Protein, Receptor, RNA, SARS, SARS-CoV-2, Smoking, Transcription, Virus

Written by
Namita Mitra
After earning a bachelor’s degree in Veterinary Sciences and Animal Health (BVSc) in 2013, Namita went on to pursue a Master of Veterinary Microbiology from GADVASU, India. Her Master’s research on the molecular and histopathological diagnosis of avian oncogenic viruses in poultry brought her two national awards. In 2013, she was conferred a doctoral degree in Animal Biotechnology that concluded with her research findings on expression profiling of apoptosis-associated genes in canine mammary tumors. Right after her graduation, Namita worked as Assistant Professor of Animal Biotechnology and taught the courses of Animal Cell Culture, Animal Genetic Engineering, and Molecular Immunology.
Source: Read Full Article
