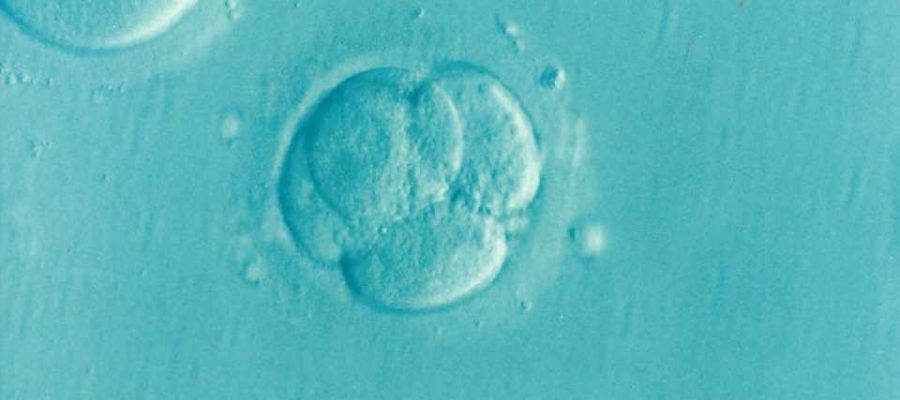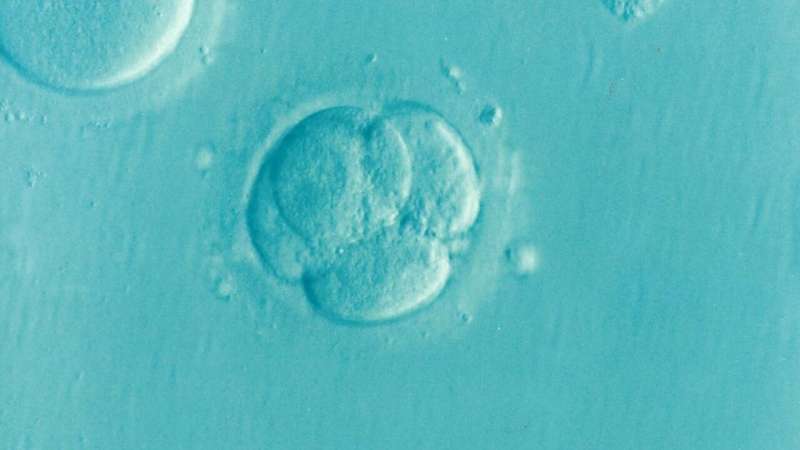
Infertility affects around 48 million couples worldwide and can have various causes. In mammals, including humans, eggs are produced in the ovary. When this process goes wrong, it can lead to female infertility. One example of this is premature ovarian insufficiency, which is characterized by problems with egg production before the age of 40. Up to 3.7% of females experience infertility as a result of this condition, and around 30% of cases are due to genetic variations.
Professor Kehkooi Kee, from Tsinghua University, China, who helped lead a new study on this topic, has been investigating this condition for several years.
“In 2019, our collaborators, Professor Li’s team, encountered a family with premature ovarian insufficiency in which changes to a gene called Eif4enif1 appeared to be responsible for the disease,” said Professor Kee.
The researchers decided to reproduce this genetic change in mice to try to understand how it affects human infertility. They show that the eggs of these mice are affected by changes to their mitochondria—the powerhouses of the cell. Their work around this new discovery is published in Development.
The researchers used CRISPR to introduce the genetic change in the mice. They allowed these mice to grow up and then compared their fertility with the fertility of mice whose DNA had not been edited. Yuxi Ding, the first author and a M.D./Ph.D. student who led the study, found that the average number of total follicles (the tiny sacs that contain developing eggs) was reduced by approximately 40% in older and genetically edited mice. The average pup number in every litter was reduced by 33%.
Importantly, when grown in a dish, about half of the eggs that were fertilized did not survive beyond the early stages of development. This demonstrated that just like the human patients, these mice were experiencing problems with fertility.
When the researchers studied the eggs from these mice under the microscope, they noticed something unusual about their mitochondria. Mitochondria produce the energy that cells—including egg cells—need. Mitochondria are usually evenly distributed throughout the egg, but the mitochondria in eggs from mice with the genetic variation were clustered together.
“We were actually surprised by the differences in the mitochondria,” said Professor Kee. “At the time we were doing this research, a link between Eif4enif1 and mitochondria had not been seen before.”
It seems likely that these misbehaving mitochondria are contributing to the fertility problems in these mice, leading the researchers to propose that restoring proper mitochondrial behavior might improve fertility.
This study provides direction for future research in human infertility, such as establishing whether mitochondrial defects are also found in the eggs of human patients with premature ovarian insufficiency and whether these same mitochondrial defects are observed in embryos after the eggs are fertilized. In addition, testing whether restoring the normal distribution of mitochondria improves fertility could become a new treatment strategy.
“Our research suggests that rescuing oocyte mitochondria abnormality could be a potential therapeutic target for clinical infertility patients with genetic variants,” says Professor Kee.
More information:
Yuxi Ding et al, Eif4enif1 haploinsufficiency disrupts oocyte mitochondrial dynamics and leads to subfertility, Development (2023). DOI: 10.1242/dev.202151
Journal information:
Development
Source: Read Full Article