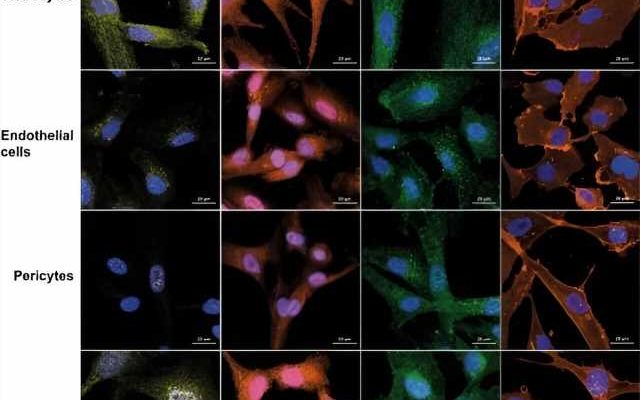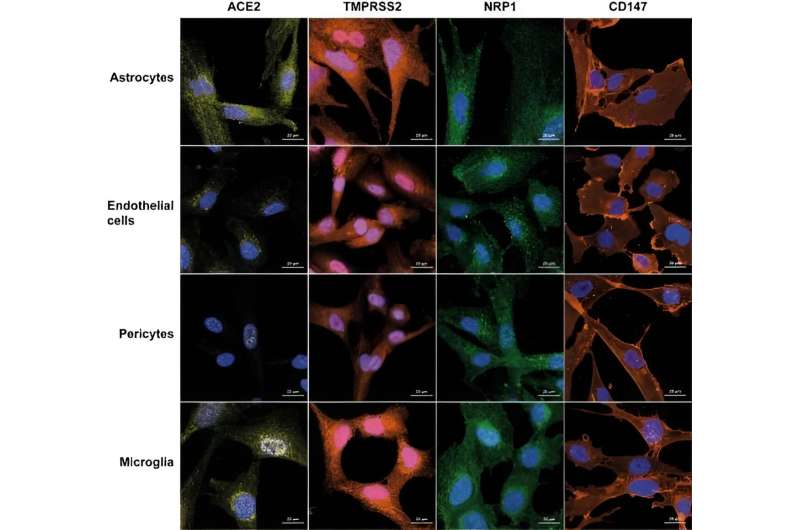
Researchers at the Francis Crick Institute have shown in lab-based experiments that variants of SARS-CoV-2, the virus that causes COVID-19, can affect the blood-brain barrier and damage brain cells in different ways. This work gives clues as to how the virus could enter the brain, but doesn’t take into account the protection that people have acquired through vaccination.
COVID-19 causes respiratory symptoms, but can also affect the brain, with many people experiencing ‘brain fog’ or cognitive deficits, and some developing neurological conditions after infection.
Researchers aren’t completely sure how the virus enters the brain, and the main theories are that it enters through the nerves that transmit smell or through the blood-brain barrier, a selective barrier that separates the brain and the bloodstream.
In this research, published in Journal of Neuroinflammation, brain cells and a 3D model of a blood-brain barrier were exposed to different strains of SARS-CoV-2: wild-type (the original variant from Wuhan), alpha, beta, delta, eta and omicron. The brain cells investigated were pericytes, astrocytes, endothelial cells and microglia—cells that support nerve cells and control how permissible the blood-brain barrier is to allowing molecules and cells to cross.
The researchers showed that all variants caused stress to brain cells, stopping them from working as well, but exactly which cells were affected depended on the variant. The wild-type virus killed all cell types except astrocytes, whereas Alpha and Beta only killed pericytes, and omicron killed endothelial cells and pericytes.
The researchers also looked at how well the variants crossed the model blood-brain barrier. The wild-type virus and to a lesser extent omicron were able to disrupt the integrity of the barrier while the other variants could not. The researchers have proposed that the virus damaging the barrier and increasing its permeability could lead to immune cells entering the brain and inducing inflammation.
Omicron killed the endothelial cells lining the barrier, but it looked like the permeability of the blood-brain barrier remained more intact, suggesting the other cells in the barrier may work to protect it. The team also found that all the variants apart from the Eta variant negatively affected junctions at the barrier—structures which connect neighboring cells and usually prevent molecules from passing between the cells—which may also influence its effectiveness.
The researchers also looked at how the variants affected the concentration of glutamate, a chemical in the brain that transmits messages from cell to cell. The amount of glutamate is tightly controlled, as too much or too little is harmful. The research showed that all the variants except Beta affected the amount of glutamate in the brain, either increasing it to potentially toxic amounts or decreasing it, reducing the ability of the cells to carry messages.
Alize Proust, senior laboratory research scientist in the Tuberculosis Laboratory at the Crick, and first author, said, “There’s been lots of speculation about how COVID-19 caused neurological symptoms, and whether it happens indirectly via inflammation, or if the virus can directly attack brain cells or cross the barrier. We’ve shown that it can do both of these in the lab, but the effects are variant-specific.”
“This has implications for what we’ve already seen. People can develop neurological disorders after a COVID-19 infection, sometimes months after. As we age, our ability to generate new neurons diminishes, so if the virus kills some brain cells, this could speed up the start of a disease affecting the brain. Our work could be important for clinicians to understand what type of damage a variant is causing, and to look for specific treatments.”
This work could form the basis for future research into the impact of the virus on the brain in humans, and whether vaccination protects against the effects seen in this research.
More information:
Alizé Proust et al, Differential effects of SARS-CoV-2 variants on central nervous system cells and blood–brain barrier functions, Journal of Neuroinflammation (2023). DOI: 10.1186/s12974-023-02861-3
Journal information:
Journal of Neuroinflammation
Source: Read Full Article