Researchers are gaining new insights into neurological diseases by studying circular RNAs (circRNAs) in brain cells. A new study by investigators from the Brigham and Women’s Hospital identified over 11,000 distinct RNA circles that characterized brain cells implicated in Parkinson’s disease and Alzheimer’s disease. Their results are published in Nature Communications.
“Circular RNA has long been cast aside as junk, but we believe it has an important role in programming human brain cells and synapses,” said corresponding author Clemens Scherzer, MD, of the Department of Neurology and the American Parkinson Disease Association Center for Advanced Parkinson Research at Brigham. “We found that these circular RNAs were produced in large quantities by brain cells, including those associated with Parkinson’s and Alzheimer’s.”
Scherzer and colleagues laser-captured neurons from 190 frozen postmortem human brain samples, including some non-neuronal cells for comparison. Then, they used ultra-deep, total RNA sequencing to study the exact sequences of genetic code found in the circular RNAs within these two cell types.
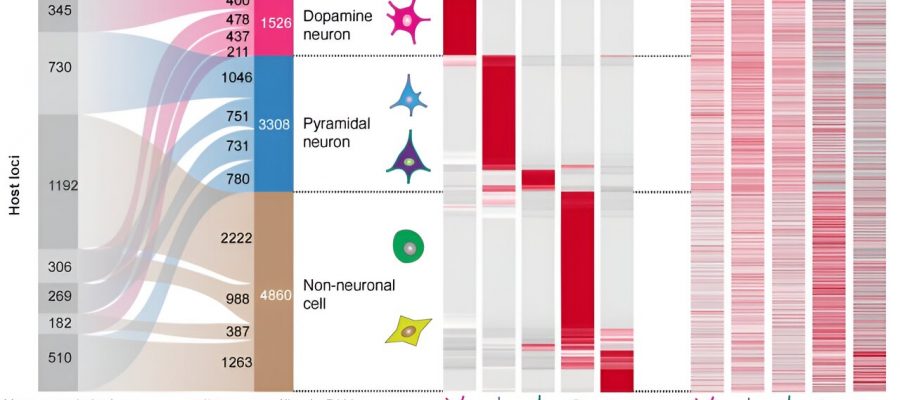
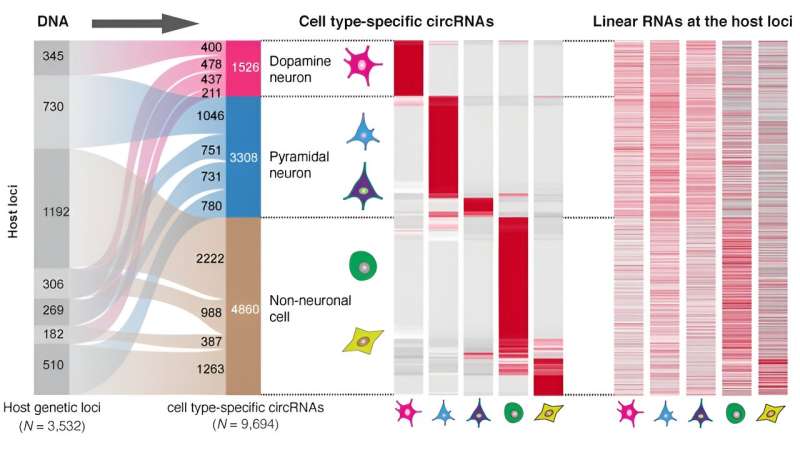
They found that 61% of all synaptic circRNAs they characterized were associated with brain disorders. Notably, they found 4,834 cell-type specific circular RNAs in dopamine and pyramidal neurons, two highly functioning brain cells. Dopamine neurons control movement, mood, and motivation while pyramidal neurons play an important role in memory and language.
“It was surprising that the circular RNAs rather than the linear RNAs produced from these gene locations defined neuron identity,” said the first author Xianjun Dong, Ph.D., an assistant professor in the Department of Neurology and the Genomics and Bioinformatics Hub at the Brigham. “circRNA diversity provides finely tuned, cell type-specific information that is not explained by the corresponding linear RNAs from the same gene.”
Degeneration of these dopamine and pyramidal neurons plays a key role in the development of neurological disorders. When researchers investigated this connection further, they found that a surprising number of Parkinson’s and Alzheimer’s genes produced circular RNA. For example, expression of one circRNA produced from the Parkinson’s gene DNAJC6 was reduced in vulnerable dopamine neurons even prior to symptom onset.

“Naturally occurring circRNAs have the potential to serve as biomarkers for specific brain cells implicated in early, prodromal stages of a disease,” Scherzer said. “Circular RNAs cannot easily be broken down, making them a powerful tool as reporters and for delivering therapies. They could be rewritten synthetically and harnessed as future digital RNA medicines.”
The team identified that genes associated with different diseases produced circRNAs in particular cell types. For example, addiction-associated genes gave rise to circRNAs in dopamine neurons, autism-associated genes in pyramidal neurons, and cancer associated genes in non-neuronal cells.
Limitations of the current study include an incomplete understanding of how this complex RNA machinery specifies neuron and synapse identity. Future research can investigate how these circRNAs arise and function and survey additional genetic regulators that govern their behavior.
Still, current findings provide the most comprehensive analysis of circRNAs in human brain cells to-date and suggest they can be leveraged for RNA diagnostics and medicines used to treat neurological conditions.
“The discovery of circular RNAs changes our understanding of the molecular mechanisms behind neurodegenerative disorders,” Dong said. “Circular RNAs are much more durable than linear RNAs and hold promise as RNA therapies and RNA biomarkers.”
More information:
Xianjun Dong et al, Circular RNAs in the human brain are tailored to neuron identity and neuropsychiatric disease, Nature Communications (2023). DOI: 10.1038/s41467-023-40348-0. www.nature.com/articles/s41467-023-40348-0
Journal information:
Nature Communications
Source: Read Full Article