A diverse array of bacteria live in the human mouth as part of a vital ecosystem known as the oral microbiome. Virginia Tech researchers have discovered that one of these common bacteria can leave the mouth and potentially cause existing cancer cells in other parts of the body to spread.
These bacteria are believed to predominantly travel through the blood to different sites in the body where they are associated with serious infections of the brain, liver, and heart; preterm birth in pregnant women; and are present in high levels in colon tumors. Poor oral hygiene could cause the bacteria to migrate to other parts of the body where cancers exist. Also, evidence exists for a link between severe gum disease and colorectal cancer.
“Our team’s discovery shows that infection with these bacteria initiates cancer cell migration,” said Daniel Slade, who is an assistant professor in the Department of Biochemistry in the College of Agriculture and Life Sciences, and an affiliated researcher in the Fralin Life Sciences Institute. “This is vital information because 90 percent of cancer-related deaths result from nonprimary tumors or sites that have metastasized to somewhere else in the body.”
The findings were published July 21 as the cover story in Science Signaling, which is produced by the American Association for the Advancement of Science.
Since 2012, multiple studies have shown this bacterium, Fusobacterium nucleatum, directly invades colon tumors, but questions remained as to how this bacterium is contributing to cancer.
A 2017 study showed that when human colon tumors containing F. nucleatum are put into a mouse, cancer cells containing live bacteria will break off and reattach in the liver, providing the first evidence that F. nucleatum could be directly involved in causing the spread of cancer cells throughout the body.
To address the potential of F. nucleatum driving metastasis, Virginia Tech researchers asked the broad question: How do human cells respond when colon cancer cells are infected with F. nucleatum? Their findings provide a deeper understanding of the critical role bacteria can play in cancer.
Origins of the project
The relatively benign nature of F. nucleatum initially intrigued Slade and his team of researchers. At first glance, Fusobacterium nucleatum appears quite unremarkable and lives in harmony with other bacteria under the gums in the oral microbiome. Despite its role as a common bacterium in the mouth, the correlations with colon cancer were too strong to ignore.
“Dan convinced me that this bacterium was a viable research direction as a bacterium that could directly influence the behavior of cancer cells,” said Scott Verbridge, a member of the team, associate professor in the Virginia Tech Department of Biomedical Engineering and Mechanics in the College of Engineering, and principal investigator of the Laboratory of Integrative Tumor Ecology. “He had developed the ability to genetically modify this bacterium. He had some amazing technology to culture this bacterium with cancer cells that was beyond anything that we could do in my lab.”
According to Slade and his team, there is no evidence that this bacterium is directly initiating cancer. Also, this bacterium does not appear to be releasing molecules that are causing the cancer cells to migrate.
Instead, F. nucleatum sticks to and even enters cancer cells using the protein Fap2, which docks with sugars overrepresented on the surface of cancer cells. This in turn causes cancer cells to release two proteins known as IL-8 and CXCL1, which are members of the cytokine protein family that play critical roles in immune system activation against infections.
Strikingly, the cytokine combination of IL-8 and CXCL1 was previously shown in multiple studies to induce the spread of cancer cells. However, Slade and his team believe this is the first example of a tumor-associated bacterium producing this distinct cytokine combination.
These cytokines released by an infected cell then can talk back to the same cell or those signals can be sent out to other cancer cells, immune cells, and various other cell types that surround a tumor. In essence, one infected cell could be affecting multiple neighboring cells, so there doesn’t have to be a widespread infection within a tumor for it to be influencing a large surrounding area.
In addition to IL-8 and CXCL1 contributing to cellular migration or metastasis, they are also potent immune cell attractants, which can lead to inflammation; a hallmark of cancer. The attraction and subsequent infection of immune cells known as neutrophils and macrophages by F. nucleatum could in turn lead to additional pro-cancerous proteins being released, which Slade and colleagues show in this work. A key contributor to the team in understanding the interactions of F. nucleatum with immune cells was Liwu Li, a professor in the Department of Biological Sciences and an affiliated researcher in the Fralin Life Sciences Institute.
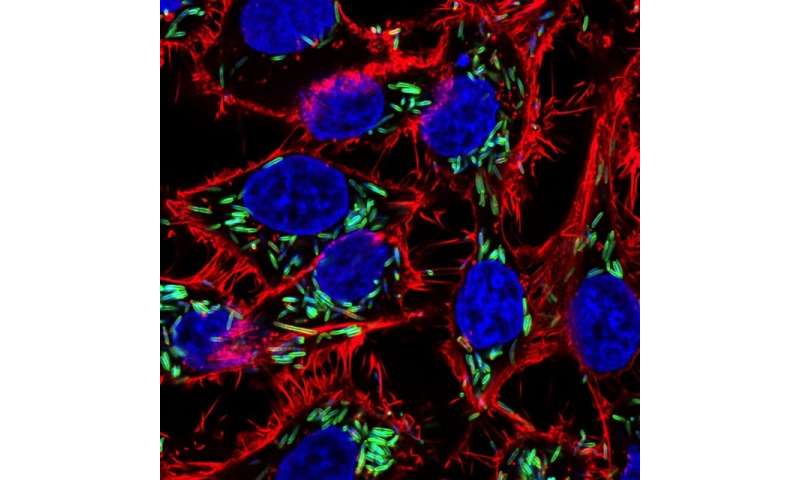
F. nucleatum, a common bacteria that can leave the mouth and potentially cause existing cancer cells in other parts of the body to spread, pictured inside of colon cancer cells.
Eyes on the future
The long-term goal of Slade and his team is to advance cancer treatment by addressing the role bacteria play in disease, which could be a critical piece that has been missing from the puzzle.
Finding pro-metastatic human proteins that are released by cancer cells upon bacterial infections has opened the door for future research. These results provide an insight into potentially blocking the secretion of cytokines to combat metastasis induced by bacteria. This is an attractive alternative to using antibiotics to kill F. nucleatum, which could also clear beneficial bacteria.
“We need to know if there are other important bacteria that could be working in synergy with F. nucleatum to drive cancer. We need to understand the physiological role of these bacteria as we can’t just go about clearing them from the body because we need them for some situations. Oftentimes, bacteria are needed for chemotherapy to be fully effective,” Verbridge said.
“I also think it’s interesting to ask if the bacteria are causing this cellular migration as a way to get around in the human body. There could be a selective advantage for any infectious agent, a virus or bacteria, that could get inside of a host cell and migrate,” Verbridge said. This could be particularly important for F. nucleatum as it is classified as a nonmotile bacterium; one that does not possess the ability to move through a lack of molecular appendages like flagella that drive movement.
Source: Read Full Article
