Treatment with the recently approved, minimally invasive Optilume BPH device improves urinary symptoms while preserving sexual function in men with benign prostatic hyperplasia (BPH), concludes a randomized trial in the September issue of The Journal of Urology.
“The next-generation Optilume BPH Catheter System offers a safe and effective new, minimally invasive treatment for BPH, reducing urinary obstruction with a low rate of sexual or other adverse effects, in a simple outpatient procedure,” comments lead author Steven A. Kaplan, MD, of Icahn School of Medicine at Mount Sinai, New York.
Sham-controlled trial helps define Optilume’s benefits and safety
Benign prostatic hyperplasia is a very common condition, estimated to affect up to 80% of men by age 80 years. It occurs when aging-related enlargement of the prostate gland causes lower urinary tract symptoms, such as frequent and difficult urination.
Available treatments include medications and surgery. Both have limitations, including a risk of problems with sexual functioning after surgery. Various minimally invasive treatments have been developed to bridge the gap between these options, with mixed results.
The Optilume BPH Catheter System offers a new type of minimally invasive treatment, featuring a dual mechanical and pharmacological mechanism of action. In the Optilume procedure, an uncoated balloon catheter is used to open a passage between the lateral lobes of the prostate (anterior commissurotomy).
A second balloon, coated with the antiproliferative drug paclitaxel, is then deployed to further widen the opening. The addition of paclitaxel is thought to prevent continued enlargement of the prostate and prevent re-fusion of the lateral lobes. The Optilume BPH system was approved by the US Food and Drug Administration earlier this year.
The new trial, called PINNACLE, included 148 men with symptomatic BPH all had urinary flow obstruction of about 30% due to impingement by the enlarged prostate. The patients, average age 65 years, were enrolled at 18 sites in the United States and Canada
100 patients were assigned to undergo active treatment with Optilume. The rest underwent a sham procedure to mimic Optilume treatment. Neither the patients nor the researchers evaluating outcomes were aware of which treatment the patient received through 12 months of follow-up. Men assigned to the sham procedure had the opportunity to undergo active treatment later on.
Lasting improvement in BPH symptoms—No adverse effects on sexual function
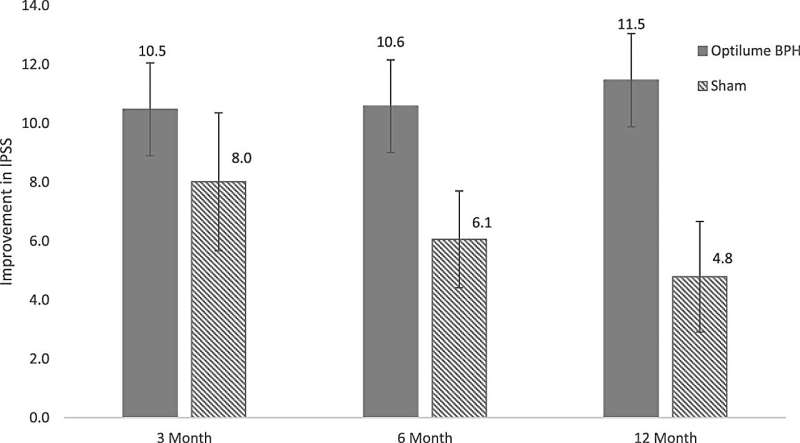
Men undergoing active Optilume treatment had greater improvement in BPH-related symptoms, as shown by the standard International Prostate Symptom Score (IPSS). From an initial value of about 24 (on a range from 0 to 35), IPSS score decreased by a median of 11.5 points with Optilume versus 8.0 points with sham treatment.
The improvement was maintained through 12 months’ follow-up in the Optilume group, but not in the sham group. About three-fourths of patients undergoing Optilume treatment had at least 30% improvement on the IPSS, compared to one-third of the sham group.
The Optilume group also had a dramatic increase in urine flow rate, greater than with previous minimally invasive treatments; and significant improvement in scores for quality of life. Adverse effects were generally mild to moderate. Importantly, sexual function was not adversely affected, including erectile and ejaculatory function.
Treatment with the Optilume BPH Catheter System is a “straightforward procedure” that can be performed in an outpatient or office setting, with simple sedation and pain control. Dr. Kaplan and coauthors conclude: “This minimally invasive treatment represents an attractive option to patients looking to maintain sexual function while achieving durable symptom relief and improved flow.”
More information:
Steven A. Kaplan et al, The PINNACLE Study: A Double-blind, Randomized, Sham-controlled Study Evaluating the Optilume BPH Catheter System for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia, Journal of Urology (2023). DOI: 10.1097/JU.0000000000003568
Journal information:
Journal of Urology
Source: Read Full Article