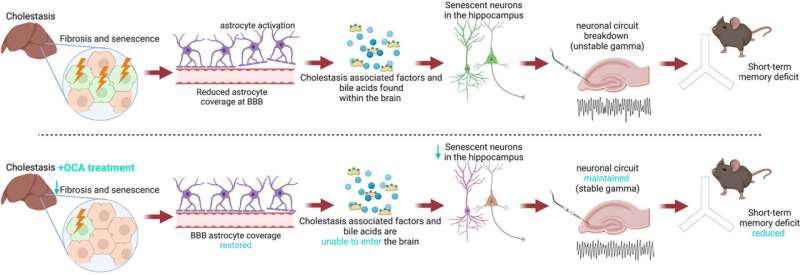
Patients with cholestatic liver disease such as primary biliary cholangitis (PBC) can experience significant impairment of bile flow and may develop neurological symptoms including fatigue and cognitive decline. Little is known about why these symptoms develop in some patients, and there is no current therapy.
In a new study in The American Journal of Pathology, investigators provide new insights into why cholestasis causes cognitive issues. They also found that obeticholic acid, a drug already used to safely treat PBC patients who do not respond well to first-line treatment, can reverse cognitive impairment.
“Difficulty in concentration and impaired short-term memory have long been associated with advanced stages of liver disease, but we now know these symptoms are a leading cause of impaired quality of life for patients at any disease stage,” said co-lead investigators Fiona Oakley, Ph.D., Newcastle Fibrosis Research Group, Biosciences Institute, Newcastle University, UK, and David E.J. Jones, MD, Ph.D., Liver Unit, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, and Translational and Clinical Research Institute, Newcastle University, UK.
“Our research was motivated by a desire to improve the lives of these patients by increasing our understanding of the mechanisms underlying these problems and use that information to identify badly needed treatment approaches.”
Using an established rodent model of bile duct ligation-induced cholestasis, the investigators first explored the extent of cognitive impairment of the type seen in human cholestatic patients.
“The importance of assessing clinical features such as short-term memory was intended to potentially tie the work to the human patient experience and how it can be improved by therapy,” explained Dr. Oakley. Cholestatic and control mice underwent a Y-maze test to evaluate spatial and short-term memory.
They displayed short-term memory and cognitive deficits compared to the controls. Pathology analysis of the brains of cholestatic mice found significant brain changes, including loss of blood brain barrier integrity, abnormality in hippocampal function, and strikingly, senescence or deterioration of neurons associated with aging.
Next, the investigators looked at whether drugs commonly used to treat PBC were effective in reversing these processes in the mice. Neither the first-line treatment for PBC, ursodeoxycholic acid (UDCA), nor bezafibrate, a second line treatment increasingly used, significantly improved short-term memory or reversed neuronal senescence.
In contrast, obeticholic acid (OCA), a second-line PBC therapy and the most potent anti-cholestatic agent approved for use in PBC to date, reversed cognitive impairment, restored blood brain barrier integrity, normalized hippocampal function, and reversed neuronal as well as liver senescence.
To extend the in vivo animal model findings to a human model, human neural cells were co-cultured with serum from both cholestatic mice and human PBC patients. Cholestatic serum induced senescence in the human cultured neurons, and, as in the mouse model, only OCA reversed the effect.
The investigators explained that the mouse model could not distinguish between an indirect effect, where OCA reduces cholestatic injury thereby mitigating the factors that lead to neurological symptoms, and a direct effect of OCA on the neurons. Their findings strongly suggest that there is a pro-senescent entity in the serum of PBC patients, and that OCA therefore works on neurons to reverse this effect.
The investigators note that neuronal senescence is an issue that arises in many diseases unrelated to cholestasis. “Our findings suggest an obvious route to treating a significant symptom in cholestatic disease for which there is no current therapy,” Dr. Oakley and Dr. Jones said. “Whether the anti-senescent effects of OCA extend to other conditions as well is a tantalizing question and one we are embarking on addressing.”
More information:
Lucy M.V. Gee et al, Anti–Cholestatic Therapy with Obeticholic Acid Improves Short-Term Memory in Bile Duct–Ligated Mice, The American Journal of Pathology (2022). DOI: 10.1016/j.ajpath.2022.09.005
Journal information:
American Journal of Pathology
Source: Read Full Article
