New findings by UT Southwestern researchers promote understanding of how one of the most commonly mutated genetic drivers of cancer passes signals that cause the disease.
The study, published in Nature Structural & Molecular Biology, focuses on a family of proteins called RAS, which is mutated in 20 to 25% of all cancers, especially in lethal cancers such as pancreatic, colorectal and lung cancers.
“A framework to develop RAS inhibitor strategies is badly needed because recently approved RAS inhibitors such as sotorasib only work against one specific mutation, and many other RAS mutations also cause cancer,” said Kenneth Westover, M.D., Ph.D., Associate Professor of Radiation Oncology and Biochemistry, member of the Chemistry and Cancer Research Program in the UT Southwestern Harold C. Simmons Comprehensive Cancer Center, and an author of the study. “This work sets the stage for development of new targeted RAS inhibitors to address major drivers of lethal cancers, such as pancreatic and colon cancer.”
Starting in 2012, Dr. Westover’s lab worked with the Dana-Farber Cancer Institute to develop drugs that bind to a specific RAS mutant where a glycine amino acid at position 12 in the RAS protein is changed to a cysteine, the so-called KRAS G12C.
“Cysteine is a distinctive amino acid that allows us to irreversibly attach drugs using special chemistries. Other major cancer-associated RAS mutations do not give us the same foothold,” Dr. Westover said.
His lab’s work helped propel the field that saw approval of one KRAS G12C inhibitor, sotorasib, in May. Approval of an analogous drug, adagrasib, is widely anticipated.
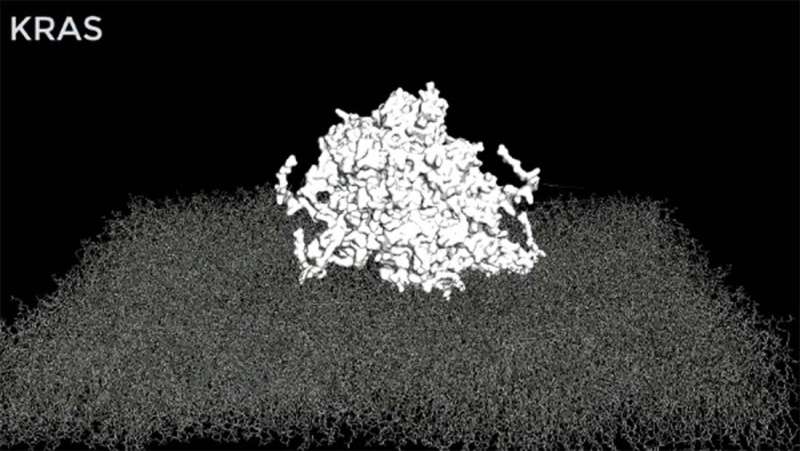
In the latest study, the Westover lab sought to understand how cancer-causing RAS mutants pass inappropriate signals from the surface of the cell to the cell nucleus. The formation of large protein clusters as part of the mechanism was known, but the clusters’ structure was unknown. Dr. Westover and collaborators used computer simulations to arrive at an atomistic structural model of a RAS assembly and validated the model using biological systems.
“This structural model is now available to the wider RAS research community. We hope it will enable researchers to test new ideas about how RAS works in normal physiology and new strategies for targeting cancer-causing RAS mutations,” said Carlos L. Arteaga, M.D., Director of the Simmons Cancer Center.
Source: Read Full Article
