A biologic therapy for very young children with a moderate to severe form of a common skin condition has been shown to be safe and effective in an international trial which involved University of Manchester clinical scientists working within the Clinical Trials Facility at Royal Manchester Children’s Hospital.
Sponsored by the Biotech companies Regeneron and Sanofi, the study of dupilumab in poorly controlled eczema is the first large-scale randomized double-blind trial of a monoclonal antibody for any skin disease in patients aged six months to six years.
The study, published in The Lancet showed that the drug greatly improved the severity of the condition, reducing skin itch and pain within two weeks.
It also significantly improved patients’ sleep, and the quality of life of patients and their parents.
Doctors expect that the international study of 162 patients will ensure this treatment is approved for British children soon, following its adoption in the United States in June this year.
Principal Investigator for Manchester and academic pediatrician Dr. Peter Arkwright, has been investigating the use of dupilumab in children with severe eczema— also known as atopic dermatitis—at Royal Manchester Children Hospital’s Clinical Research Facility (CRF) since August 2015.
Although the therapy is already licensed in the U.K. for adults and children aged 6 to 18, researchers see the positive results for young children as the icing on the cake.
The patients in the trial were enrolled from 31 hospitals, clinics, and academic institutions in Europe and North America from June 2020, to February 2021.
A total of 83 patients were given a subcutaneous injection of dupilumab, and 79 a placebo every four weeks as well as continuing standard therapy with low-potency steroid cream for 16 weeks. Independent assessors scored the level of inflammation.
They found:
- 28% of patients receiving dupilumab achieved a global skin score of 0 or 1 indicating clear or almost clear skin at week 16.
- 53% of the patients experienced a 75% improvement from the baseline in Eczema Area and Severity Index (EASI) at week 16.
- Conjunctivitis was slightly more frequent with dupilumab than placebo (5% vs 0%), however no dupilumab-related adverse events were serious or led to treatment discontinuation.
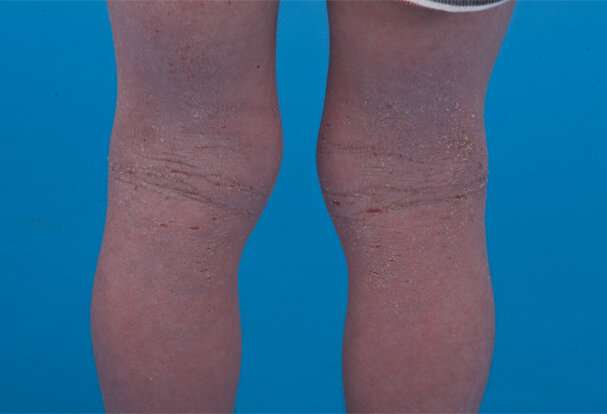
Eczema is a chronic inflammatory skin disease with prevalence of 20% or higher in children younger than six years.
It is characterized by an itchy red rash particularly on the face and the bends of the elbows and knees, as well as an increased risk of skin infection.
Dr. Arkwright said, “Young children and infants who have moderate-to-severe eczema have a substantially reduced quality of life. It’s also incredibly stressful for their families, particularly as children’s sleep is so disturbed.
“The fact that infants and young children with moderate-to-severe eczema are inadequately controlled with creams means they have a high unmet medical need. We’re so delighted that dupilumab has provided clinically meaningful improvement, with an acceptable safety profile.
Source: Read Full Article
