A team of Harvard and Sloan Kettering scientists has developed compounds that can target and degrade proteins associated with acute myeloid leukemia (AML) and nearly doubled the life expectancy of mice with cancer in laboratory tests.
Because the degradation of these proteins blocks cell growth and delays leukemia progression, their method of multitargeted protein degradation holds promise for the creation and delivery of therapeutic molecules to inhibit more types of cancer.
“Developing new molecules that degrade new targets for different types of cancer is a massive area of ongoing research in the pharmaceutical industry as well as in academia,” said Christina Woo, one of the paper’s senior authors and associate professor of chemical biology. “By altering the targets that are degraded, we gain the ability to treat new cancers.”
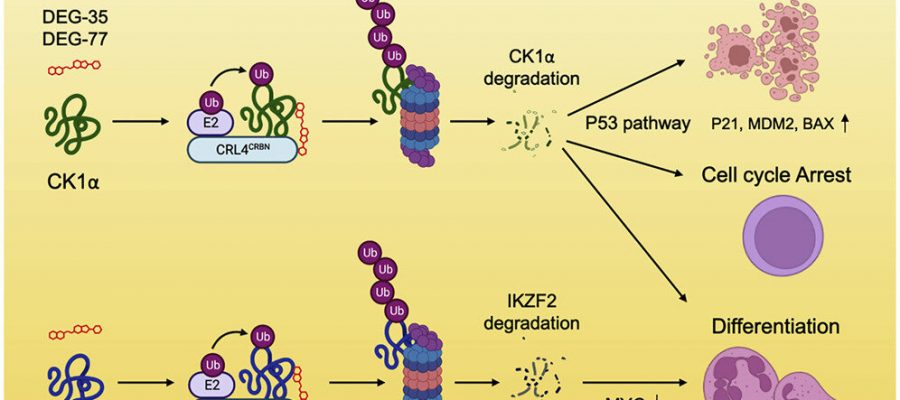
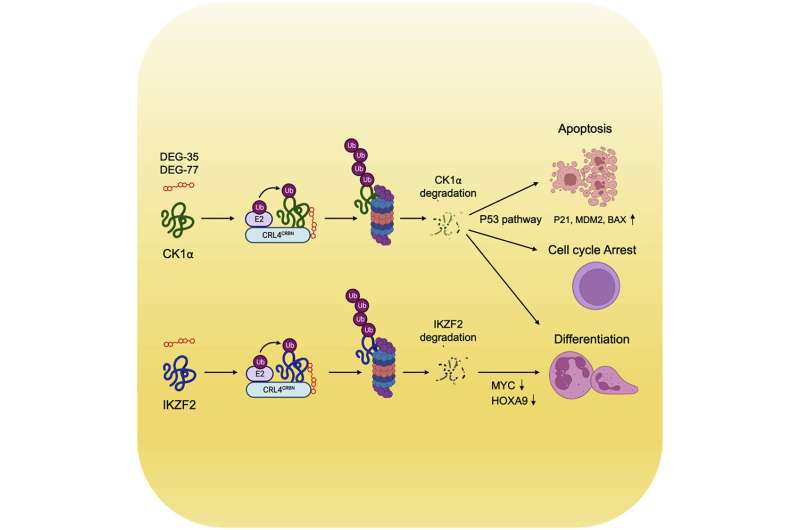
In the paper, published in Cancer Cell, the researchers detailed how they developed degrader molecules DEG-35 and DEG-77, which identify and break down proteins contributing to cancer. The authors sought to degrade two proteins, IKZF2 and CK1α, which have been difficult to target selectively, let alone simultaneously. By testing specific chemical features to alter the degradation scope of the molecules, the team was able to successfully dial out one protein and dial in another to craft their effective degraders.
The work was undertaken by several members of Woo’s lab, including David K. Miyamoto, Nicole Curnutt, Nathan L. Tran, and Wenqing Xu, in collaboration with Michael Kharas, a member in molecular pharmacology at Sloan Kettering Institute, and members of his lab.

Proteins associated with cancer and the molecules that can degrade them have significant therapeutic potential. Molecules that degrade targets through cereblon, a protein encoded by the CRBN gene, are widely used to treat multiple myeloma. However, existing protein degradation strategies have been limited when dealing with aggressive cancers like AML.
The research team tailored a class of molecules that act like a “molecular glue,” sealing protein-protein interactions together to promote the degradation of one of the targets. In doing so, these compounds created a new space for targeting cancer-promoting proteins that were once thought to be inaccessible.
With their expertise in targeted protein degradation and this class of molecules, Woo’s lab generated a series of compounds and tested them in tissue culture before sending the best ones to the Kharas Lab. In doing so, they developed promising molecules that successfully revealed molecular details about interactions on the protein’s binding surface.
The Kharas Lab, which has done extensive research in myeloid leukemia, tested the molecules in cells and then performed the in vivo studies by injecting the compounds into mice with cancer. One injection of DEG-77 in mice led to the degradation of IKZF2 and CK1α in bone marrow and leukemic cells in the spleen. After 24 hours, leukemia cells in the mice’s blood, bone marrow, and spleen underwent increased apoptosis and myeloid differentiation, reducing their colony-forming ability. DEG-77-treated mice had a significantly prolonged survival of 66 days compared to 35 days without loss of total body weight.
Going forward, Woo and Kharas are optimistic that that their compounds and protein-degradation strategies will be useful in developing drugs to treat an expanded variety of cancers.
“In the future we would like to go from a tool compound, a sort of proof of concept, to an orally available compound that will be tested in people,” Kharas said. “This is really one of the most exciting approaches in the lab because it’s got the most potential to being put into patients.”
“We are already looking at how we can use the same targets for other cancers,” Woo said. “Our group is starting to look at ovarian cancer with additional collaborators.”
More information:
Christina M Woo, Dual IKZF2 and CK1? degrader targets acute myeloid leukemia cells, Cancer Cell (2023). DOI: 10.1016/j.ccell.2023.02.010. www.cell.com/cancer-cell/fullt … 1535-6108(23)00038-7
Journal information:
Cancer Cell
Source: Read Full Article