Teams at the Institut Pasteur, CNRS, Vaccine Research Institute (VRI) and Université de Paris have discovered a new function of anti-HIV-1 antibodies by applying cutting-edge microscopy techniques to in vitro viral cultures. The scientists found that certain antibodies already known for effectively targeting HIV-1 envelope (Env) protein can prevent infected cells from releasing viral particles, thus halting viral spread. The antibodies are Y-shaped, enabling them to attach themselves between the infected cell and viral particles or directly between viral particles. This chain composed of antibodies and viral particles prevents viral spread. These findings demonstrate that these powerful antibodies exhibit different antiviral activities in addition to neutralization. The study is published in the February 2, 2022 issue of Nature Communications.
Broadly neutralizing antibodies (bNAbs) targeting virus envelope (Env) protein have significant potential for treating HIV-1. They were initially identified in rare cases of patients whose serum was capable of inhibiting numerous HIV strains. These antibodies exhibit multiple antiviral activities. As well as neutralizing the virus, i.e. preventing it from infecting new cells, they also kill infected cells. Consequently, they are referred to as polyfunctional molecules. It is necessary to fully understand the scope of these antiviral activities in order to use existing antibodies more effectively or refine the selection criteria for new antibodies. It is moreover useful to further investigate the polyfunctionality of anti-HIV-1 antibodies in order to improve our understanding of the role played by antibodies and thus tackle other viral infections.
Initially, teams at the Institut Pasteur, CNRS, VRI and Université de Paris sought to determine whether antibodies were capable of preventing infected cells from producing viral particles. To that end, they cultured CD4 T cells (HIV’s natural target) in vitro with various antibodies for 24 hours. They subsequently measured the quantity of viral particles produced by the cells in the culture medium and the quantity of viral particles remaining in the cells. As a result of these experiments, the scientists were able to demonstrate that certain antibodies increased virus quantity in cells but reduced it in the culture medium. This intriguing finding led them to believe that certain antibodies impeded the release of viral particles without preventing their production.
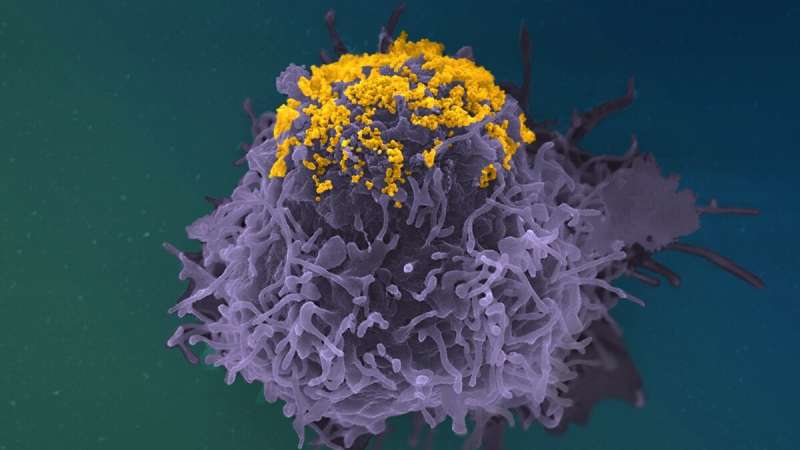
To test this theory, the scientists used various microscopy techniques to observe cells’ production of viral particles. They initially examined the cells by fluorescence microscopy, a technique used to differentiate virus proteins. This enabled them to demonstrate that infected cells accumulate large quantities of mature viral protein. This finding suggests that full viral particles accumulate in cells. To determine the precise location of these viral particles, the scientists subsequently used scanning electron microscopy to observe the surface of infected cells. “Using this method, we observed that these antibodies (bNAbs) prompt an accumulation of viral particles at the surface of cells, forming clusters and highly atypical structures (see illustration),” comments Timothée Bruel, co-last author of the study and scientist in the Virus and Immunity Unit at the Institut Pasteur.
Next, the scientists combined a transmission electron microscopy technique with immunogold labeling. This enabled them to demonstrate that antibodies interpose themselves between viral particles and the infected cell, forming a chain cluster. Experiments with mutant antibodies subsequently demonstrated that the antibodies’ Y shape creates this clustered structure. Their arms are capable of linking two viruses, or one virus to the infected cell membrane, and their attachment points are sufficiently strong to prompt this phenomenon.
“We have demonstrated that only the most powerful antibodies tether viral particles at the surface of infected cells. Trapped viral particles can no longer infect new cells,” concludes Olivier Schwartz, co-last author of the study and Head of the Virus and Immunity Unit at the Institut Pasteur.
Source: Read Full Article
