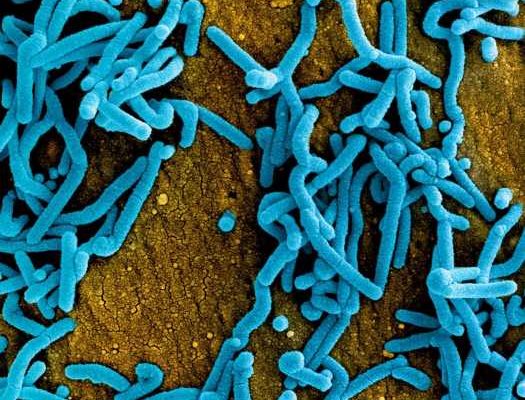
A newly published paper in The Lancet shows that an experimental vaccine against Marburg virus (MARV) was safe and induced an immune response in a small, first-in-human clinical trial. The vaccine, developed by researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, could someday be an important tool to respond to Marburg virus outbreaks.
This first-in-human, Phase 1 study tested an experimental MARV vaccine candidate, known as cAd3-Marburg, which was developed at NIAID’s Vaccine Research Center (VRC). This vaccine uses a modified chimpanzee adenovirus called cAd3, which can no longer replicate or infect cells, and displays a glycoprotein found on the surface of MARV to induce immune responses against the virus. The cAd3 vaccine platform demonstrated a good safety profile in prior clinical trials when used in investigational Ebola virus and Sudan virus vaccines developed by the VRC.
MARV, a filovirus in the same family as Ebola virus, causes a rapidly progressive febrile illness that leads to shock and death in a large proportion of infected individuals. Many scientists think that MARV disease outbreaks in humans begin when the virus makes the jump from its primary animal host, which is likely to be certain chronically infected bats in sub-Saharan Africa. The symptoms of MARV disease are akin to those seen with Ebola virus disease and can include fever, headache, chills, rash, abdominal pain, vomiting, and diarrhea. As the disease progresses, patients may suffer from multiple organ dysfunction, delirium, and significant bleeding from the gastrointestinal tract or other sites that may result in death.
No approved vaccines or specific therapies are available for MARV disease, aside from supportive care. While some experimental vaccines have previously been tested, none have proven to be both highly effective and to provide durable protection. In areas of Africa where a vaccine for Marburg is most needed, a single-dose vaccine that could protect recipients over a long period of time would be a crucial part of quelling outbreaks.
In this study, 40 healthy adult volunteers were enrolled at the Walter Reed Army Institute of Research Clinical Trials Center in Silver Spring, Maryland. They received a single dose of either a low dose of the vaccine (1×1010 particle units) or a higher dose (1×1011 particle units). For safety, the volunteers were enrolled in a dose-escalation plan. Three participants received the lower dose. Then, when they did not exhibit severe adverse reactions after the first seven days, the trial proceeded to enroll the remaining 17 volunteers. The same procedure was also used for the higher dose group. Volunteers were monitored for adverse reactions to the investigational vaccine and evaluated at regular intervals for 48 weeks to track their immune responses.
The trial’s safety results were encouraging: There were no serious adverse events, and the experimental vaccine was well-tolerated. One participant in the higher dose group developed a fever following vaccination, but it resolved by the following day. In addition, the investigational vaccine appeared to induce strong, long-lasting immunity to the MARV glycoprotein: 95% of participants in the trial exhibited a robust antibody response after vaccination, and 70% maintained that response for more than 48 weeks.
Plans are in place to conduct further trials of the cAd3-Marburg vaccine in Ghana, Kenya, Uganda, and the United States. If additional data supports the promising results seen in the Phase 1 trial, the cAd3-Marburg virus vaccine could someday be used in emergency responses to MARV outbreaks.
More information:
Melinda J Hamer et al, Safety, tolerability, and immunogenicity of the chimpanzee adenovirus type 3-vectored Marburg virus (cAd3-Marburg) vaccine in healthy adults in the USA: a first-in-human, phase 1, open-label, dose-escalation trial, The Lancet (2023). DOI: 10.1016/S0140-6736(22)02400-X
Journal information:
The Lancet
Source: Read Full Article