Predicting which patients will respond well to treatment is a quandary that has plagued the field of cancer immunotherapy for more than four decades. Now, researchers at the Johns Hopkins Kimmel Cancer Center and its Bloomberg~Kimmel Institute for Cancer Immunotherapy are one step closer to solving that problem. In a small study, they successfully trained a machine learning algorithm to predict, in hindsight, which patients with melanoma would respond to treatment and which would not respond.
The open-source program, DeepTCR, proved valuable as a predictive clinical tool, but it also functioned as a powerful instructor, teaching the researchers about the biological mechanisms underlying patients’ responses to immunotherapy.
“DeepTCR’s predictive power is exciting,” says John-William Sidhom, M.D., Ph.D., first author of the study, “but what I found more fascinating is that we were able to view what the model learned about the immune system’s response to immunotherapy. We can now exploit that information to develop more robust models, and possibly better treatment approaches, for many diseases, even those outside of oncology.”
A summary of the research was published in the journal Science Advances.
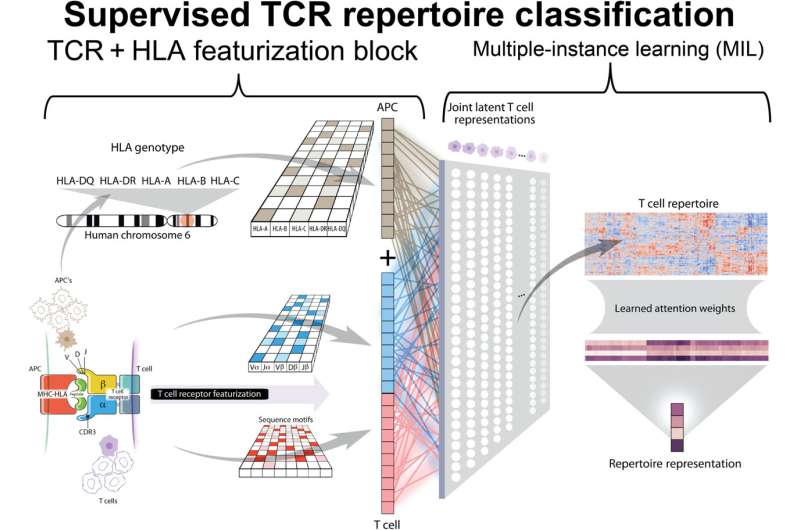
DeepTCR was developed at the Johns Hopkins University School of Medicine by Sidhom when he was an M.D./Ph.D. student. It uses deep learning, a form of artificial intelligence, to recognize patterns in large volumes of data. In this case, the data is the amino acid sequences of proteins called T cell receptors (TCRs).
TCRs sit on the exterior of the immune system’s T cells, waiting to be engaged by a protein from an enemy: cancer, bacteria or viruses. TCRs are like locks that can only be opened by a single key. The T cell’s exterior is studded with many TCRs, but they are all identical and are all opened by the same enemy key. Not knowing which enemies are present, many different T cells roam the body. When a TCR is activated, its T cell releases molecules to kill the enemy, and it clones itself to fortify the response.
Unfortunately, some tumor cells develop ways of blocking the T cells’ response, even though the TCRs have been activated. Current immunotherapy drugs, known as checkpoint inhibitors, consist of proteins that stymie this capacity in tumors, causing T cells to respond to cancer. However, these drugs help only a minority of patients.
In the current study, Sidhom, now a resident, used materials collected during the CheckMate 038 clinical trial that tested the efficacy of one immunotherapy drug (nivolumab) compared to a combination of two (nivolumab and ipilimumab) for 43 patients with inoperable melanoma. Biopsies of the tumors, containing an array of infiltrating T cells, were taken before and during treatment.
In the CheckMate study, no significant differences were seen in patients treated with the single drug versus the two-drug combination. Some patients in both groups responded and others did not.
Using a well-established protocol, Sidhom used high-tech genetic sequencing to discover the TCR repertoire surrounding each tumor by determining the type and number of TCRs in each biopsy. He then fed that data to the DeepTCR program and told it which data sets belonged to responders versus nonresponders. Then the algorithm looked for patterns.
The researchers first asked if there were differences before treatment between the TCR repertoires of immunotherapy in responders and nonresponders. The differences that the algorithm identified were as predictive of patient response as known biomarkers—molecular characteristics of tumors used to guide therapy. However, before the algorithm can be used clinically to guide therapy, the researchers need to confirm these findings in a larger patient population.
“Precision immunotherapy based on the immune microenvironment in the tumor is critical to guide the optimal choice of treatment options for each patient,” says Drew Pardoll, M.D., Ph.D., professor of oncology and director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy.
“These DeepTCR findings define a new dimension for predicting a tumor’s response to immune checkpoint blockade by applying a novel artificial intelligence strategy to deconvolute the vast array of receptors expressed by tumor-infiltrating T cells, the key immune components responsible for direct killing of tumor cells.”
Next, Sidhom wanted to know what the differences were between responders and nonresponders. He used data from another study that linked specific TCRs (identified by their amino acid sequences) to the enemy proteins that activated them. In the data set were thousands of TCRs, and each responded to a different protein from a variety of invaders: the flu virus, the Epstein-Barr virus, the yellow fever virus and tumors.
What was found was counterintuitive: The patients who responded to the immunotherapy were those who had a higher number of virus-specific T cells in their tumors. Nonresponders had more tumor-specific T cells.
Looking at the changes in the TCR repertoires of each patient after treatment began, Sidhom learned that the nonresponders had higher turnover of T cells. “Both responders and nonresponders had about the same number of tumor-specific T cells before and during therapy,” he says.
“The identity of those T cells remained the same in the responders, but in the nonresponders, there was a different variety of T cells before and during therapy. Our hypothesis is that nonresponders had a high number of ineffective tumor-specific T cells from the start. When the immunotherapy began, their immune systems sent in a new batch of T cells, trying to find an effective one, but the dysfunction remained. On the other hand, the responders had effective T cells from the onset, but their anti-tumor activity was blocked by the tumor. When the immunotherapy began, it released the blockade and allowed them to do their job.”
“The application of the deep-learning framework in DeepTCR to characterize the TCR repertoire of T cells allows for improved stratification of patient outcomes along with model explainability in terms of identifying the predictive features,” says Alexander Baras, M.D., Ph.D., associate professor of pathology at the Johns Hopkins University School of Medicine and director of precision medicine informatics at the Johns Hopkins Kimmel Cancer Center.
Sidhom says the core of the algorithms in DeepTCR is a “neural network,” which is one of the least explainable artificial intelligence models—meaning it is difficult to learn what the model learned. “This paper shows how you can use even a neural network to extract an explanation about the biology behind its predictions,” he says. “The ability to provide ‘explainable AI’ will prove invaluable to cancer biology and many other fields.”
More information:
John-William Sidhom et al, Deep learning reveals predictive sequence concepts within immune repertoires to immunotherapy, Science Advances (2022). DOI: 10.1126/sciadv.abq5089
Journal information:
Science Advances
Source: Read Full Article
