Scientists are unraveling the complex mystery underlying how the immune system mounts a potent defense against one of the world’s most relentless killers—the deadly parasites that cause the worst form of malaria.
In two unrelated studies, both published in Science Translational Medicine, scientists arrived at the same conclusion: the immune system’s natural killer cells, among the critical first responders during parasite proliferation, are key to the control of the mosquito-borne infection.
As part of the study, scientists in Kenya turned to adult volunteers who were intentionally infected under strict clinical conditions with the deadliest species of the malaria-causing organism. The research, which was wide-ranging, assessed the infection and natural killer cell response in the volunteers.
In a separate arm of the study, the team examined blood samples from children who reside in a Kenyan region where malaria is endemic. And in a final portion of their investigation, scientists analyzed assaults on malaria parasites by natural killer cells in laboratory dishes.
These investigators, at the Center for Geographic Medicine Research in Kalifi, Kenya, make a sound argument for better defining the role of natural killer cells and their attack on one of the most formidable infectious agents in the world.
In the second study, led by scientists in California, an international team of researchers pinpointed a unique population of natural killer cells that emerge in children who have been exposed to malaria in an endemic region of Uganda.
Africa is the most severely affected continent on the planet when it comes to the mosquito-borne disease, according to the World Health Organization, which also emphasized that malaria’s spread is driven by a highly efficient carrier, the Anopheles gambiae mosquito. It’s a nighttime flyer that bites between 10 p.m. and 4 a.m. when people are usually asleep and unlikely to swat and crush them.
The Anopheles gambiae is often called the world’s deadliest animal.
Worse, the mosquito carries the most lethal malaria parasite, Plasmodium falciparum. Among the four species of malaria parasites that infect humans, P. falciparum is associated with severe infection and high mortality. The group most adversely affected is children under the age of 5.
In 2019, the most recent year for complete statistics, an estimated 386,000 people died of malaria in Africa and 274,000 were children, according to WHO.
After years of failed attempts at vaccine development, several immunizations are emerging and one developed in the U.K. by the University of Oxford and pharmaceutical giant GlaxoSmithKline, has been approved in Ghana and Nigeria. The vaccine was designed for children between the ages of five months and three years. Fourteen investigational vaccines were studied as part of the new research in Kenya to determine their role in prompting a natural killer cell response.
“Natural killer cells are potent immune effectors,” writes Dennis Odera, lead author of the Kenyan research, describing natural killers, a class of immune system constituents that target and kill infected cells. Natural killers are so named because killing is their chief specialty, a job mediated by potent molecules contained within the killers’ lysosomes. These bodies are secretory organelles tucked within natural killers that release harsh compounds. These chemicals are set free as the rough-riding natural killers encounter their deadly targets.
Odera and colleagues noted that natural killer cells mediate immune responses against Plasmodium falciparum using multiple effector functions: Hitting the parasite with harsh chemicals and signaling the onslaught of inflammatory molecules, which literally increase the temperature, making conditions within the body difficult for the infectious organisms.
Understanding how the immune system responds to P. falciparum helps better illuminate the complex biology of the organism and its activity in the human bloodstream. Studying the active organism and its encounters with the human immune system adds new insight for developing the next generation of vaccines.
“Nine of the 14 vaccine candidates tested induced antibody-dependent natural killer cells,” added Odera, referring to antibodies that developed in response to vaccination, and which in turn, signaled natural killer activity.
Malaria parasites are protozoa that must thrive in two different hosts—female mosquitoes and humans—during their complex, three-stage life cycle. Those three stages are the ookinete, which occurs in the gut of the mosquito; the sporozoite the more mature form that is released in mosquito saliva when it bites, and the merozoite, the stage of the parasite that evolves in the human liver.
Odera and the Kenya-based team are interested in the merozoite stage of the parasite’s development because this is when the damage of malaria begins. When a female Anopheles mosquito bites a human, parasites in the sporozoite stage enter the blood and invariably travel to the liver where broods of parasites grow.
They emerge from the liver and enter erythrocytes—red blood cells—as merozoites, which continue a relentless cycle of invading more and more red cells. As merozoites burst free from erythrocytes they destroy these vital oxygen-ferrying cells in the process.
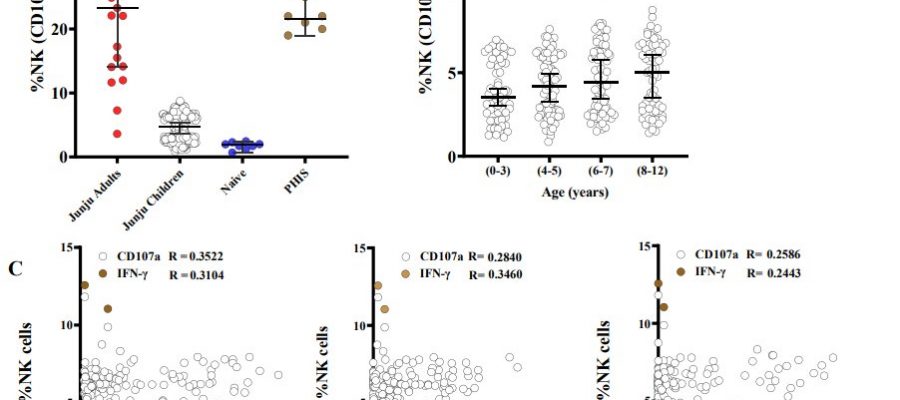
The Kenyan study covered multiple bases in an effort to understand natural killer cell activity and merozoites in the human bloodstream. Along with the controlled malaria infections of Kenyan adults, the study also analyzed of 293 blood samples from a longitudinal cohort of children living in Junju, a region of Kenya with high malaria transmission.
Additionally, there was a laboratory component of the research, which allowed Odera and colleagues to observe natural killer cells in action—how they respond to merozoites in culture. What the team saw was the biological equivalent of chemical warfare.
Natural killer cells released antimicrobial compounds through a process called degranulation. The inflammatory molecule interferon-gamma turned up the heat. Fighting malaria isn’t easy. The parasites are big—substantially larger than bacteria and multiple orders of magnitude larger than viruses. The fight is ferocious, ravaging, and ugly.
By releasing antimicrobial compounds and a flood of inflammatory molecules, such as interferon-gamma, Odera and his collaborators saw how malaria parasites can be stopped from further invading red blood cells.
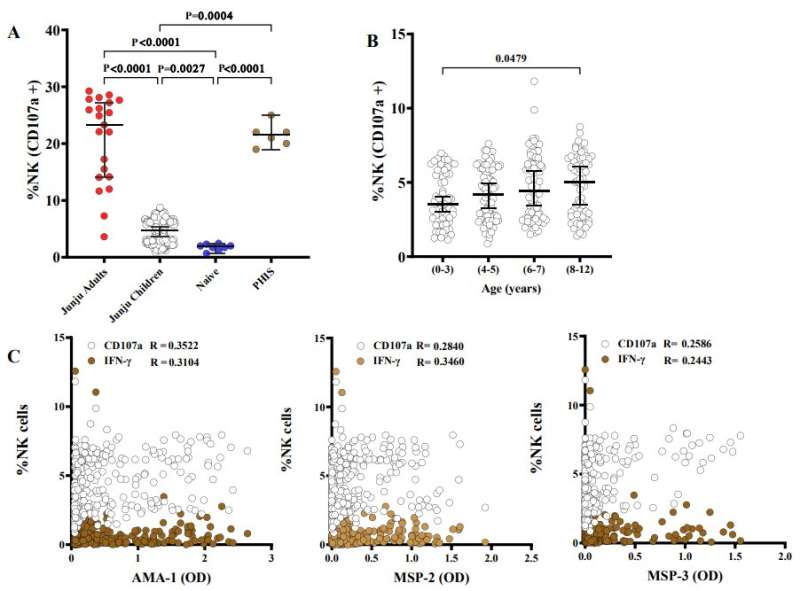
In the controlled human malaria infection part of the study, the team observed that Kenyan adults who didn’t develop fever harbored higher amounts of antibody-dependent natural killer cells than those who needed treatment. The team then examined the 293 samples from children living in Junju and found that antibody-dependent natural killer cells rose with age and spiked during infections with P. falciparum.
Higher numbers of antibody-dependent natural killer cells correlated with a lower risk of clinical malaria and symptoms, suggesting that natural killer cells play a key role in buttressing malaria immunity.
“Using multiparameter flow cytometry, we found that natural killer cells degranulate and release interferon-gamma upon stimulation with antibody-opsonized Plasmodium falciparum merozoites,” Odera noted, referring to antibodies that had tagged parasites for destruction.
“Antibody-dependent natural killer cell activity was largely strain transcending and enhanced invasion inhibition into erythrocytes,” Odera added, describing how immune system forces prevented invasion into additional red blood cells.
“Antibody-dependent natural killer cells were associated with the successful control of parasitemia after experimental malaria challenge in African adults,” Odera continued, noting that in children, “antibody-dependent natural killer cells increased with age, was boosted by concurrent P. falciparum infections, and was associated with a lower risk of clinical episodes of malaria.”
In the second study, focusing on children and malaria, the international team of scientists underscored that natural killer cell activity is key to the immune response in malaria.
Led by researchers at Stanford University, the scientific paper highlights how a special population of natural killer cells supports the immune system’s ability to protect the body from malaria parasites. “Natural killer cells likely play an important role in immunity to malaria,” asserted Maureen Ty of Stanford, lead author of a study that included researchers in Uganda and Australia.
The linchpin in their research was the identification of an atypical subset of natural killer cells, a group dubbed CD56neg. These cells expand during repeated parasite exposures.
The team studied natural killer cells in a group of 264 Ugandan children and underscored that the CD56neg subset, provide protection against symptomatic malaria. “Understanding factors that drive programming of this unique [natural killer] cell subset will help guide therapeutic translation, including enhancing vaccine-elicited protection,” Ty concluded.
More information:
Dennis O. Odera et al, Anti-merozoite antibodies induce natural killer cell effector function and are associated with immunity against malaria, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abn5993
Maureen Ty et al, Malaria-driven expansion of adaptive-like functional CD56-negative NK cells correlates with clinical immunity to malaria, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.add9012
Journal information:
Science Translational Medicine
Source: Read Full Article