Editor’s note: This is the second in a two-part series commemorating the 100-year anniversary of the first use of insulin in humans. Part 1 of this series examined the rivalry behind the discovery and use of insulin.
One hundred years ago, teenager Leonard Thompson was the first patient with type 1 diabetes to be successfully treated with insulin, granting him a reprieve from what was a certain death sentence at the time.
Since then, research has gathered pace. In the century since insulin’s discovery and first use, recombinant DNA technology has allowed for the engineering of the insulin molecule, providing numerous short- and long-acting analog versions. At the same time, technological leaps in automated insulin delivery and monitoring of blood glucose ensure more time with glucose in range and fewer life-threatening complications for those with type 1 diabetes fortunate to have access to the technology.
In spite of these advancements, there is still scope for further evolution of disease management, with the holy grail being the transplant of stem cell-derived islet cells capable of making insulin, ideally encased in some kind of protective device so that immunosuppression is not required.
Indeed, it is not unreasonable to “hope that type 1 diabetes will be a curable disease in the next 100 years,” says Elizabeth Stephens, MD, an endocrinologist who has type 1 diabetes and practices in Portland, Oregon.
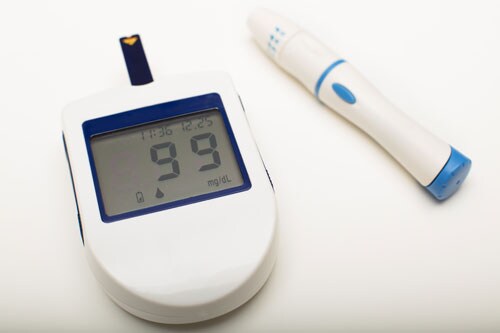
Technology now allows automated insulin delivery and monitoring of blood glucose.
Type 1 Diabetes: The Past 100 Years
The epidemiology of type 1 diabetes has shifted considerably since 1922. A century ago, given that average life expectancy in the United States was around 54 years, it was pretty much the only type of diabetes that doctors encountered. “There was some type 2 diabetes about in heavier people, but the focus was on type 1 diabetes,” notes Stephens.
Originally called juvenile diabetes because it was thought to only occur in children, “now 50% of people are diagnosed with type 1 diabetes…over [the age of] 20,” explains Stephens.
In the United States, around 1.4 million adults 20 years and older, and 187,000 children younger than aged 20, have the disease, according to data from the National Diabetes Statistics Report 2020 by the US Centers for Disease Control and Prevention (CDC).
This total represents an increase of nearly 30% from 2017.
Over the years, theories as to the cause, or trigger, for type 1 diabetes “have included cow’s milk and [viral] infections,” says Stephens.
“Most likely, there’s a genetic predisposition and some type of exposure, which creates the perfect storm to trigger disease,” she adds.
There are hints that COVID-19 might be precipitating type 1 diabetes in some people. Recently, the CDC found SARS-CoV-2 infection was associated with an increased risk for diabetes (all types) among youth, but not other acute respiratory infections. And two further studies from different parts of the world have recently identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons remain unclear.
The global CoviDiab registry has also been established to collect data on patients with COVID-19-related diabetes.
The million-dollar question: Is COVID-19 itself is propagating type 1 diabetes or unmasking a predisposition to the disease sooner? The latter might be associated with a lower type 1 diabetes rate in the future, says Partha Kar, MBBS, OBE, national specialty advisor, diabetes, for NHS England.
“Right now, we don’t know the answer. Whichever way you look at it, it is likely there will be a rise in cases, and in countries where insulin is not freely available, healthcare systems need to have supply ready because insulin is lifesaving in type 1 diabetes,” Kar emphasized.

Type 1 diabetes cases in children have increased since the start of the pandemic.
CGMs and Automated Insulin Delivery: A “Godsend”

Dr Kersten Hall
A huge change has also been seen, most notably in the past 15 to 20 years, in the technological advancements that can help those with type 1 diabetes live an easier life.
Continuous glucose monitors (CGMs) and automated ways of delivering insulin, such as smart pens and insulin pumps, have made the daily life of a person with type 1 diabetes in the Western world considerably more comfortable.
CGMs provide a constant stream of data to an app, often wirelessly in sync with the insulin pump. However, on a global level, they are only available to a lucky few.
In England, pending NICE (National Institute for Health and Care Excellence) approval, any CGM should be available to all eligible patients with type 1 diabetes within the National Health Service (NHS) from April of this year, Kar points out. In the United States, CGMs are often unaffordable and access is mostly dependent on a person’s health insurance.
Kersten Hall, PhD, a scientist and UK-based medical historian who recently wrote a book, Insulin, the Crooked Timber, uncovering the lesser-known story behind the discovery of insulin, was diagnosed with adult-onset type 1 diabetes at the age of 41. Hall had always found the finger-prick blood glucose test to be a chore but now has a CGM.
“It’s a total game changer for me: a godsend. I can’t sing its praises enough,” he remarks. “All it involves is the swipe of the phone and this provides a reading which tells me if my glucose is too low, so I eat something, or too high, so I might [go for] a run.”
Brewing Insulin at Scale
As described by Hall in his book, the journey from treating Thompson in 1922 to treating the masses began when biochemist James Collip, MD, PhD, discovered a means of purifying the animal pancreas extracts used to treat the teenager.
But production at scale presented a further challenge. This was overcome in 1924 when Eli Lilly drew on a technique used in the beer brewing process — where pH guides bitterness — to purify and manufacture large amounts of insulin.
By 1936, a range of slower-acting cattle and pig-derived insulins, the first produced by Novo Nordisk Pharmaceuticals, were developed.
However, it took 8000 lb (approximately 3600 kg) of pancreas glands from 23,500 animals to make 1 lb (0.5 kg) of insulin, so a more efficient process was badly needed.
Hall, who is a molecular biologist as well as an author, explains that the use of recombinant DNA technology to produce human insulin, as done by Genentech in the late 70s, was a key development in the story of modern insulin products. Genentech then provided synthetic human insulin for Eli Lilly to conduct clinical trials.
Human insulin most closely resembles porcine insulin structure and function, differing by only one amino acid, while human insulin differs from bovine insulin by three amino acid residues. This synthetic human insulin eliminated the allergies that the animal-derived products sometimes caused.
In the early 1980s, Eli Lilly produced Humulin, the first biosynthetic (made in Escherichia coli, hence the term, “bio”) human insulin.
This technology eventually “allowed for the alteration of specific amino acids in the sequence of the insulin protein to make insulin analogs [synthetic versions grown in Escherichia coli and genetically altered for various properties] that act faster, or more slowly, than normal human insulin. By using the slow- and fast-acting insulins in combination, a patient can control their blood sugar levels with a much greater degree of finesse and precision,” Hall explains.
Today, a whole range of insulins are available, including ultra-rapid-acting, short-acting, intermediate-acting, long-acting, ultra-long acting, and even inhaled insulin, although the latter is expensive, has been associated with side effects, and is less commonly used, according to Stephens.
Oral insulin formulations are even in the early stages of development, with candidate drugs by Generex and from the Oralis project.
“With insulin therapy, we try to reproduce the normal physiology of the healthy body and pancreas,” Stephens explains.
Insulin analogs are only made by three companies (Eli Lilly, Novo Nordisk, and Sanofi), and they are generally much more expensive than non-analog human insulin. In the UK through the NHS, they cost twice as much.
In the United States today, one of the biggest barriers to proper care of type 1 diabetes is the cost of insulin, which can limit access. With the market controlled by these three large companies, the average cost of a unit of insulin in the United States, according to RAND research, was $98.17 in January 2021, compared with $7.52 in the UK and $12.00 in Canada.
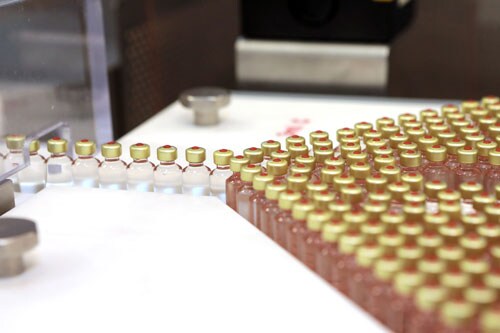
Insulin production has grown more efficient but access issues remain for many patients.
Several US states have enacted legislation capping insulin copayments to at, or under, $100 a month. But the federal Build Back Better Framework Act — which would cap copayments for insulin at $35 — currently hangs in the balance.
Alongside these moves, in 2020 the US Food and Drug Administration (FDA) approved the first interchangeable biosimilar insulin for type 1 diabetes (and insulin-dependent type 2 diabetes) in children and adults, called Semglee (Mylan Pharmaceuticals).
Biosimilars (essentially generic versions of branded insulins) are expected to be less expensive than branded analogs, but the indications so far are that they will only be around 20% cheaper.
“I totally fail to understand how the richest country in the world still has a debate about price caps, and we are looking at biosimilar markets to change the debate. This makes no sense to me at all,” stresses Kar. “For life-saving drugs, they should be funded by the state.”
Insulin also remains unaffordable for many in numerous low- and middle-income countries, where most patients pay out-of-pocket for medicines. Globally, there are estimated to be around 30 million people who need insulin but cannot afford it.
How Near to a Cure in the Coming Decades?
Looking ahead to the coming years, if not the next 100, Stephens highlights two important aspects of care.
First, the use of a CGM device in combination with an insulin pump (also known as a closed-loop system or artificial pancreas), where the CGM effectively tells the insulin pump how much insulin to automatically dispense, should revolutionize care.
A number of such closed-loop systems have recently been approved in both the United States, including systems from Medtronic and Omnipod, and Europe.
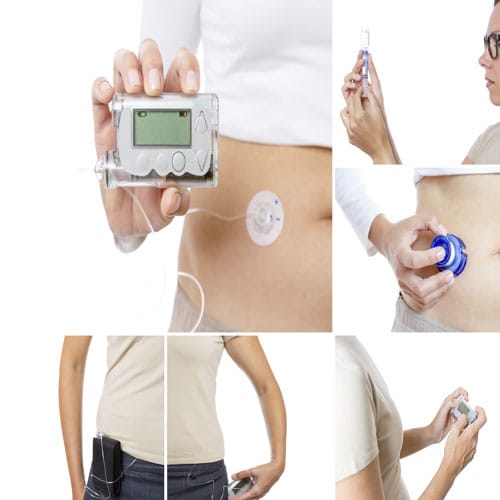
New technology, including insulin pumps, has improved insulin delivery for patients.
“I wear one of these and it’s been a life changer for me, but it doesn’t suit everyone because the technology can be cumbersome, but with time, hopefully things will become smaller and more accurate in insulin delivery,” Stephens adds.
The second advance of interest is the development and transplantation of cells that produce insulin.
Stephens explains that someone living with type 1 diabetes has a lot to think about, not least, doing the math related to insulin requirement. “If we just had cells from a pancreas that could be transplanted and would do that for us, then it would be a total game changer.”
To date, Vertex Pharmaceuticals has successfully treated one patient — who had lived with type 1 diabetes for about 40 years and had recurrent episodes of severe hypoglycemia — with an infusion of stem cell–derived differentiated islet cells into his liver. The procedure resulted in near reversal of type 1 diabetes, with his insulin dose reduced from 34 to 3 units, and his A1c falling from 8.6% to 7.2%.
And although the patient, Brian Shelton, still needs to take immunosuppressive agents to prevent rejection of the stem-cell derived islets, “it’s a whole new life,” he recently told The New York Times.
Another company called ViaCyte is also working on a similar approach.
Whether this is a cure for type 1 diabetes is still debatable, says Anne Peters, MD, of the University of Southern California, Los Angeles. “Is it true? In a word, no. But we are part of the way there, which is much closer than we were 6 months ago,” she explained in a recent video commentary.
There are also ongoing clinical trials of therapeutic interventions to prevent or delay the trajectory from presymptomatic to clinical type 1 diabetes. The most advanced is the anti-CD3 monoclonal antibody teplizumab (Tzield, Provention Bio), which was rejected by the US FDA last summer, but has since been refiled. The company expects to hear from the agency by the end of March 2022 as to whether the resubmission has been accepted.
Diabetes Specialist Nurses/Educators Keep it Human
Hall says he concurs with the late eminent UK diabetes specialist Robert Tattersall’s observation on what he considers one of the most important advances in the management and treatment of type 1 diabetes: the human touch.
Referring to Tattersall’s book, Diabetes: A Biography, Hall quoted: “If asked what innovation had made the most difference to their lives in the 1980s, patients with type 1 diabetes in England would unhesitatingly have chosen not human insulin, but the spread of diabetes specialist nurses (DSNs)…these people (mainly women) did more in the last two decades of the 20th century to improve the standard of diabetes care than any other innovation or drug.”
In the United States, DSNs were called diabetes educators until recently, when the name changed to certified diabetes care and education specialist (CDCES).
“Above all, they have humanized the service and given the patient a say in the otherwise unequal relationship with all-powerful doctors,” concludes Hall, again quoting Tattersall.
For more diabetes and endocrinology news, follow us on Twitter and Facebook.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Source: Read Full Article
