In a recently published article in the journal Cell Reports, scientists have demonstrated that individuals with or without a history of coronavirus disease 2019 (COVID-19) exhibit differential humoral and cellular immune responses 14 days after receiving mRNA-based COVID-19 vaccine developed by Pfizer/BioNTech. However, at a later time point, the responses become comparable between the two groups.
Study: Cellular and humoral functional responses after BNT162b2 mRNA vaccination differ longitudinally between naive and subjects recovered from COVID-19
Background
The COVID-19 vaccines developed within one year of the pandemic have significantly impacted the pandemic trajectory in terms of reducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and disease severity. Regarding vaccine-induced immunity, it has been documented that while COVID-19 recovered individuals are capable of inducing a robust humoral immunity after a single vaccine dose, naïve individuals require two doses of the vaccine to induce a similar response.
Regarding the duration of vaccine-induced immunity, reports are highlighting that the vaccine efficacy against SARS-CoV-2 infection may reduce with time; however, the vaccine can still protect against severe COVID-19. The waning protection against infection might be due to a gradual reduction in the levels of neutralizing antibodies with time. However, it is still uncertain how vaccine-induced cellular responses contribute to overall protection.
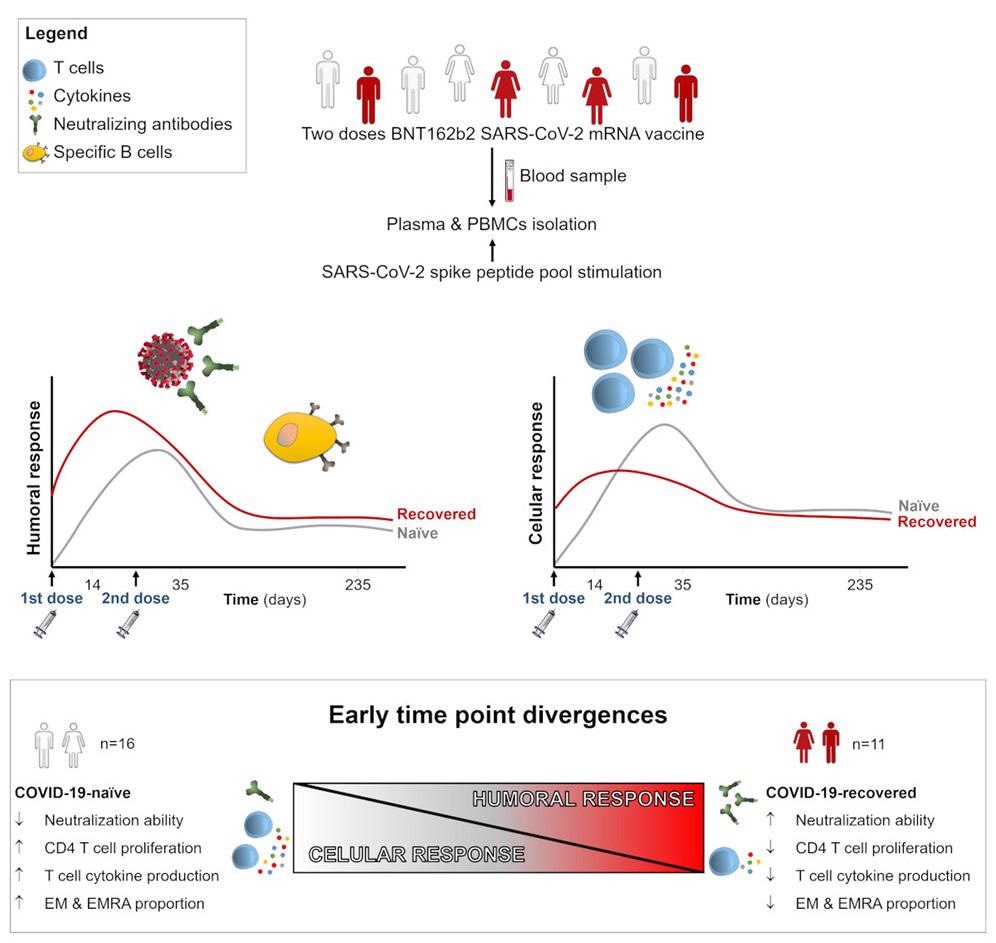
In the current study, the scientists have evaluated antibody-mediated and T cell-mediated immune responses induced by mRNA-based COVID-19 vaccine (Pfizer/BioNTech) in individuals with or without prior exposure to SARS-CoV-2.
They have assessed anti-spike antibody and T cell responses in blood samples collected at baseline (before vaccination), 14 days after the first vaccine dose, and 14 days and 8 months after the second dose.
Vaccine-induced humoral immune response
The study enrolled a total of 27 individuals who had received two doses of the Pfizer vaccine at an interval of 21 days. Of all participants, 11 had prior SARS-CoV-2 exposure and 16 were naïve.
The analysis of humoral response revealed that the first vaccine causes an induction in blood levels of anti-spike S1 and anti-spike receptor-binding domain (RBD) IgA antibodies in both naïve and COVID-19 recovered (convalescent) individuals. However, the antibody levels were higher in convalescent individuals after the first dose than in naïve individuals. In contrast, an equivalent antibody titer was observed between both groups after the second vaccine dose. Regarding IgG-specific anti-spike, anti-S1, and anti-RBD antibodies, convalescent individuals showed higher antibody titers than naïve individuals at all tested time points.
The analysis of neutralization potency of plasma revealed that a single vaccine is sufficient to induce neutralizing titers in convalescent individuals. In contrast, naïve individuals required two vaccine doses to achieve the same. Although convalescent individuals showed comparatively higher neutralizing titers 14 days after the second dose, an equivalent neutralization potency was observed between both groups 8 months after the second vaccination.
Vaccine-induced cellular immune response
The analysis of cytokine and chemokine production by T cells revealed that naïve individuals have higher spike-specific cytokine production (IL-2) by both CD4+ and CD8+ T cells and higher activation and proliferation of CD4+ T cells than convalescent individuals after 14 days of the second vaccination. However, both groups showed a comparable spike-specific T cell response 8 months after the second vaccination.
The analysis of T cell subpopulations revealed a robust induction of IL-2 production in effector T cell populations (effector memory cells and CD45RA-re-expressing effector memory cells) in naïve individuals after 14 days of the second vaccination. However, a gradual reduction of effector T cell response was observed with time.
Considering all tested immunological parameters, an inverse correlation was observed between the humoral and cellular immune responses 14 days after the second vaccination. Specifically, a significant inverse correlation was observed between IgG antibody production and virus neutralization and CD4+ T cell proliferation and IL-2 production at an early time point after vaccination. However, no such correlation was observed at a later time point, i.e., 8 months after the second vaccination.
Study significance
The study findings indicate that naïve and COVID-19 recovered individuals induce differential humoral and cellular immune responses against SARS-CoV-2 at early phases after full immunization with Pfizer-developed COVID-19 vaccine. However, at later time points, these responses become comparable between both groups.
- Roberto Lozano-Rodríguez. 2021. Cellular and humoral functional responses after BNT162b2 mRNA vaccination differ longitudinally between naive and subjects recovered from COVID-19. Cell Reports. https://www.sciencedirect.com/science/article/pii/S2211124721017447
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Antibodies, Antibody, Blood, CD4, Cell, Cell Proliferation, Chemokine, Coronavirus, Coronavirus Disease COVID-19, covid-19, Cytokine, Efficacy, Immune Response, immunity, Immunization, Pandemic, Proliferation, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus

Written by
Dr. Sanchari Sinha Dutta
Dr. Sanchari Sinha Dutta is a science communicator who believes in spreading the power of science in every corner of the world. She has a Bachelor of Science (B.Sc.) degree and a Master's of Science (M.Sc.) in biology and human physiology. Following her Master's degree, Sanchari went on to study a Ph.D. in human physiology. She has authored more than 10 original research articles, all of which have been published in world renowned international journals.
Source: Read Full Article
