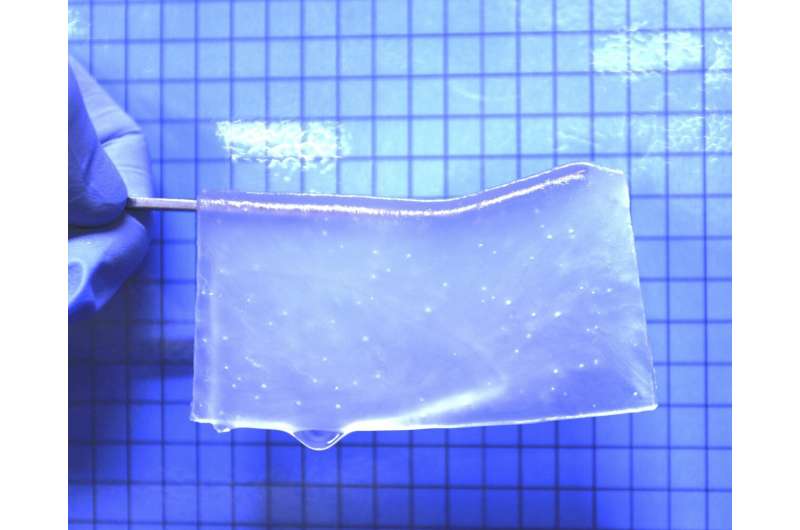
Synthetic hydrogels were shown to provide an effective scaffold for neuronal tissue growth in areas of brain damage, providing a possible approach for brain tissue reconstruction.
While growing brains may sound like something out of a science fiction movie, a cross-disciplinary team of researchers at Hokkaido University have made a step in that direction. They used hydrogel materials, in combination with neural stem cells, to grow new brain tissue. This is important because when tissue in our brain is damaged, the neuronal tissue does not have the same regenerative capacity as other parts of our body, such as skin.
The first step for researchers was to develop a hydrogel material in which neural stem cells could survive. They found that a neutral gel made with equal parts positively and negatively charged monomers resulted in the best cell adhesion. Researchers then adjusted the ratios of crosslinker molecules to achieve a stiffness similar to that of brain tissue; pores were then created in the gel in which cells could be cultured.
“When I saw the 3D structure of the porous hydrogels that my colleague Tomáš showed in a meeting, I thought they could be utilized in regenerative treatments as a scaffold for growing nerve cells,” recalled lead author Satoshi Tanikawa.
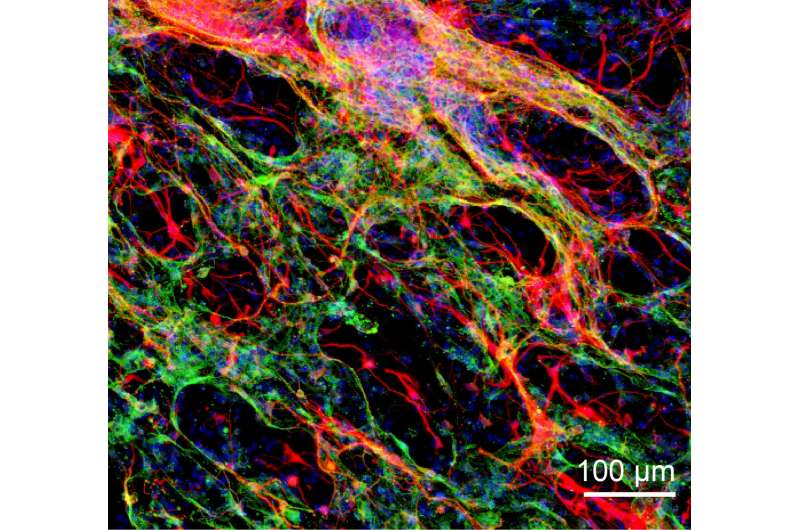
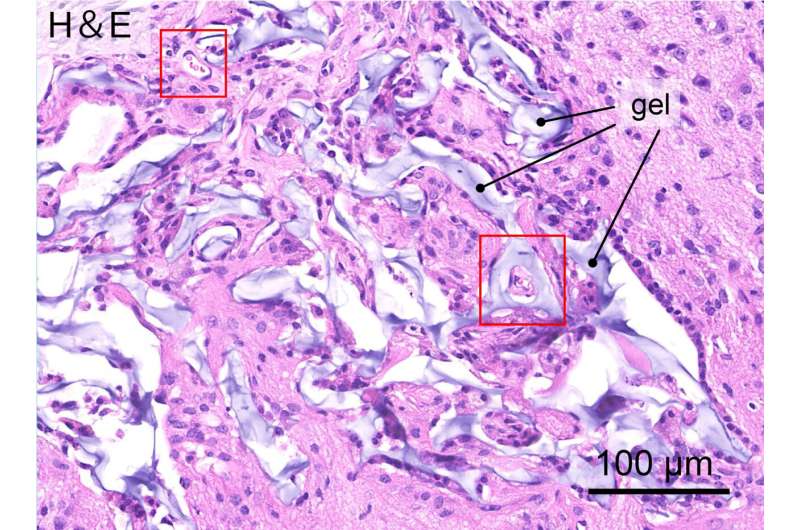
Once the gels were optimized, they were soaked in a growth factor serum to encourage blood vessel growth, and then they were implanted in damaged areas of the brain in a mouse model. After three weeks, researchers found that immune cells and neuronal cells from the surrounding host brain tissue had entered the hydrogel and that blood vessels had grown.
At this point, researchers injected neural stem cells into the hydrogel. After 40 days, stem cell survival rate was high, and some had differentiated into new astrocyte cells or neuronal cells. It was observed that host cells infiltrated the hydrogel, while some new neuronal cells from the hydrogel migrated to the surrounding brain tissue, showing some degree of integration between the hydrogel and host brain tissue.
The stepwise nature of the process was key, as implanting the hydrogel and transplanting the neural stem cells at the same time proved unsuccessful. This study marks an important step toward developing therapies involving brain tissue regeneration; the next steps involve studying the optimal transplant timing and the effect of the inflammatory response on transplanted cells.
“Conditions affecting blood vessels in the brain, such as cerebral infarction, are a major disease,” commented Tanikawa. “They not only have a high mortality rate but those that survive struggle with severe after-effects. I think this research will become the foundation for medical treatments that could help such patients.”
The study is published in the journal Scientific Reports.
More information:
Satoshi Tanikawa et al, Engineering of an electrically charged hydrogel implanted into a traumatic brain injury model for stepwise neuronal tissue reconstruction, Scientific Reports (2023). DOI: 10.1038/s41598-023-28870-z
Journal information:
Scientific Reports
Source: Read Full Article
