In a new study, published July 12 in Nature, researchers have produced the most detailed and comprehensive human Heart Cell Atlas to date, including the specialized tissue of the cardiac conduction system—where the heartbeat originates.
The multi-center team is led by the Wellcome Sanger Institute and the National Heart and Lung Institute at Imperial College London, and has also presented a new drug-repurposing computational tool called Drug2cell, which can provide insights into the effects of drugs on heart rate.
This study is part of the international Human Cell Atlas (HCA) initiative, which is mapping every cell type in the human body, to transform our understanding of health and disease, and will form the foundation for a fully integrated HCA Human Heart Cell Atlas.
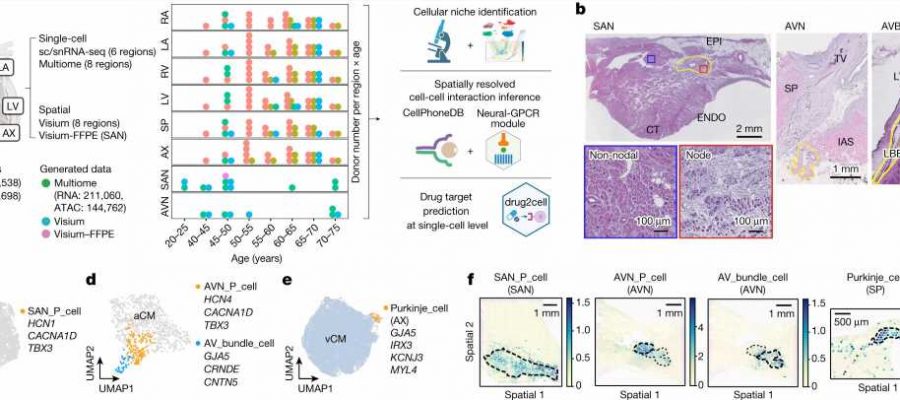
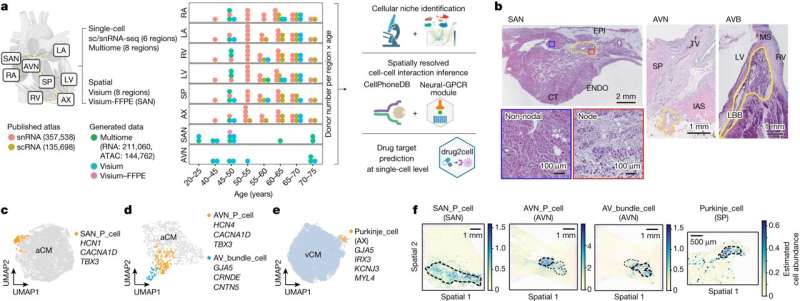
Charting eight regions of the human heart, the work describes 75 different cell states including the cells of the cardiac conduction system—the group of cells responsible for the heartbeat—not understood at such a detailed level in humans before. The human cardiac conduction system, the heart’s “wiring,” sends electrical impulses from the top to the bottom of the heart and coordinates the heartbeat.
By using spatial transcriptomics, which gives a “map” of where cells sit within a tissue, researchers were also able to understand how these cells communicate with each other for the first time. This map acts as a molecular guidebook, showing what healthy cells look like, and providing a crucial reference to understand what goes wrong in disease. The findings will help understand diseases such as those affecting the heart rhythm.
The assembly of a Human Heart Cell Atlas is key given that cardiovascular diseases are the leading cause of death globally. Around 20,000 electronic pacemakers are implanted each year in the U.K. for these disorders. These can be ineffective and are prone to complications and side-effects. Understanding the biology of the cells of the conduction system and how they differ from muscle cells paves the way to therapies to boost cardiac health and develop targeted treatments for arrhythmias.
The team also presents a new computational tool called Drug2cell. The tool can predict drug targets as well as drug side effects. It leverages single-cell profiles and the 19 million drug-target interactions in the EMBL-EBI ChEMBL database.
Unexpectedly, this tool identified that pacemaker cells express the target of certain medications, such as GLP1 drugs, which are used for diabetes and weight loss and are known to increase the heart rate as a side-effect, the mechanism of which was unclear. This study suggests that the increase in heart rate might be partly due to a direct action of these drugs on pacemaker cells, a finding the team also showed in an experimental stem cell model of pacemaker cells.
Dr. James Cranley, joint first author, a cardiologist specializing in heart rhythm disorders and Ph.D. student at the Wellcome Sanger Institute, said, “The cardiac conduction system is critical for the regular and coordinated beating of our hearts, yet the cells which make it up are poorly understood. This study sheds new light by defining the profiles of these cells, as well as the multicellular niches they inhabit. This deeper understanding opens the door to better, targeted anti-arrhythmic therapies in the future.”
Dr. Kazumasa Kanemaru, joint first author and Postdoctoral Fellow in the Gene Expression Genomics team at the Wellcome Sanger Institute, said, “The mechanism of activating and suppressing pacemaker cell genes is not clear, especially in humans. This is important for improving cell therapy to facilitate the production of pacemaker cells or to prevent the excessive spontaneous firing of cells. By understanding these cells at an individual genetic level, we can potentially develop new ways to improve heart treatments.”
The study unearthed an unexpected discovery: a close relationship between conduction system cells and glial cells. Glial cells are part of the nervous system and are traditionally found in the brain. They have been explored very little in the heart. This research suggests that glial cells are in physical contact with conduction system cells and may play an important supporting role: communicating with the pacemaker cells, guiding nerve endings to them, and supporting their release of glutamate, a neurotransmitter.
Another key finding of the study is an immune structure on the heart’s outer surface. This contains plasma cells, which release antibodies into the space around the heart to prevent infection from the nearby lungs. The researchers also identified a cellular niche enriching for a hormone that could be interpreted as an early warning sign of heart failure.
Dr. Michela Noseda, senior Lecturer in Cardiac Molecular Pathology at the National Heart and Lung Institute, Imperial College London, a Coordinator of the Human Cell Atlas Heart BioNetwork and a lead author, said, “We often don’t fully know what impact a new treatment will have on the heart and its electrical impulses—this can mean a drug is withdrawn or fails to make it to the market. Our team developed the Drug2cell platform to improve how we evaluate new treatments and how they can affect our hearts, and potentially other tissues too. This could provide us with an invaluable tool to identify new drugs which target specific cells, as well as help to predict any potential side-effects early on in drug development.”
Professor Metin Avkiran, Associate Medical Director at the British Heart Foundation, said, “Using cutting-edge technologies, this research provides further intricate detail about the cells that make up specialized regions of the human heart and how those cells communicate with each other. The new findings on the heart’s electrical conduction system and its regulation are likely to open up new approaches to preventing and treating rhythm disturbances that can impair the heart’s function and may even become life-threatening.
“International collaboration is key to scientific progress. This impactful study and other discoveries from the broader Human Cell Atlas initiative are excellent examples of what can be achieved when the international research community works together across borders. Our combined efforts can ultimately produce better outcomes for patients worldwide.”
Dr. Sarah Teichmann, a senior author of the study from the Wellcome Sanger Institute and co-chair of the Human Cell Atlas Organizing Committee, said, “This Heart Cell Atlas reveals cardiac microanatomy in unprecedented detail, including the cardiac conduction system that enables each heartbeat, and is a valuable reference for studying heart disease and designing potential therapeutics. An important contribution to the global Human Cell Atlas initiative, which is mapping every cell type in the body to understand health and disease, it will form the foundation for a fully integrated HCA Human Heart Cell Atlas. In addition, our suite of computational methods will help identify possibilities for repurposing existing drugs to treat diseases in other tissues.”
More information:
Kazumasa Kanemaru et al, Spatially resolved multiomics of human cardiac niches, Nature (2023). DOI: 10.1038/s41586-023-06311-1
Journal information:
Nature
Source: Read Full Article