A validated Symptom Burden Questionnaire that will help medical researchers unravel the complexities of Long COVID and develop or test new treatments is now available for translation into hundreds of languages, with support for inclusion on digital platforms, thanks to a partnership agreement between the University of Birmingham, U.K., and Mapi Research Trust.
Globally over 100 million people are currently thought to be affected by Long COVID. However, attempts to study this complex condition have been stifled by a broad and variable constellation of symptoms that can affect many organs in the body—and patients had told researchers that existing questionnaires did not fully capture the breadth of their symptoms.
The Symptom Burden Questionnaire for Long COVID (SBQ-LC ) was developed by a team from the University of Birmingham’s Centre for Patient-Reported Outcomes Research to address this challenge.
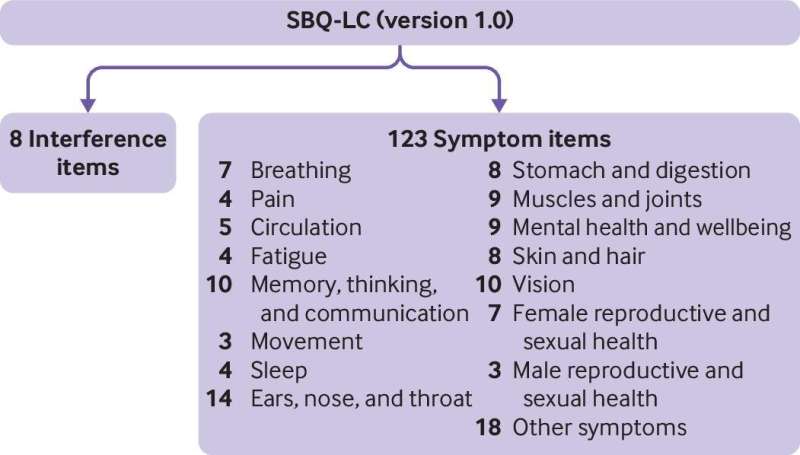
Designed according to international best practice and regulatory guidance, the SBQ-LC uses modern methods to capture symptoms from 16 domains (categories of symptoms), and one scale that measures symptom impact on daily life, and has already been licensed for use in over 50 countries throughout the world.
While the best known Long COVID symptoms are fatigue and brain fog, up-to-date research shows the symptom list also includes nausea and vomiting, incontinence, erectile dysfunction, and hair loss, and the World Health Organisation has recently urged countries to expedite research into recovery and rehabilitation.
Birmingham’s partnership with Mapi Research Trust means the questionnaire can be e-migrated, translated, and cross-culturally validated through rigorous linguistic validation processes to support international research efforts to understand more about the disease, identify treatment options, and improve the clinical management of people affected by it throughout the world.
Caroline Anfray, Director of Collaborations with Clinical Outcome Assessment (COA) developers and Copyright holders at Mapi Research Trust explains, “High-quality supplemental materials for COAs ensures consistency across versions and translations. Reliable user guidance is essential to assure the integrity of COAs and the accuracy of the results they gather.”
“Mapi Research Trust is committed to the highest standards in clinical trial resources, and our mission on behalf of the University of Birmingham will ensure that the SBQ-LC is used consistently and appropriately by research teams across languages, countries and technology.”
Dr. Sarah Hughes, Research Fellow at Birmingham’s Institute of Applied Health Research, led the project team that designed and validated the SBQ-LC. She said, “The questionnaire is designed for a comprehensive capture of symptoms, while not being burdensome for patients who can complete it either on paper, or via a digital App.”
The full list of domains on the SBQ-LC comprises: breathing, pain, circulation, fatigue, memory, thinking and communication, movement, sleep, ear, nose and throat, stomach and digestion, muscles and joints, mental health and well-being, skin and hair, eyes, reproductive health (male/female), and other symptoms.
Dr. Hughes added, “While the underlying causes of Long COVID are not yet clear, it remains important to record, accurately and reliably, the fullest roster of symptoms, so we can create datasets of patient experience that can be compared or consolidated.”
“The SBQ provides a modular approach, with the option of collecting comprehensive data, or only data relating to specific clusters of symptoms. Conversion of raw scores to a common metric (0-100) allows easy comparison of scores across the different symptom domains, delivers a real-world picture of the burden of disease and its impact on everyday life, and its scores may be used to support regulatory decisions around the approval of new therapies for Long COVID.”
The findings are published in the journal BMJ.
More information:
Sarah E Hughes et al, Development and validation of the symptom burden questionnaire for long covid (SBQ-LC): Rasch analysis, BMJ (2022). DOI: 10.1136/bmj-2022-070230
Journal information:
British Medical Journal (BMJ)
Source: Read Full Article
